Water is a very weak acid, with insufficient proton concentration to start the electrophilic addition reaction. It is necessary to add an acid (H2SO4 ) to the medium for the reaction to take place.
This reaction is also known as hydration of alkenes and generates alcohols.
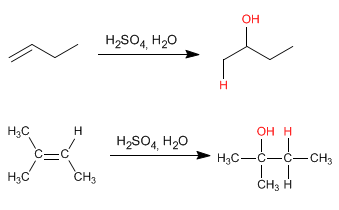
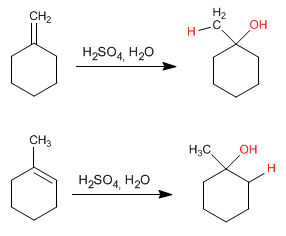
This reaction is carried out with dilute sulfuric acid 50% sulfuric/H2O and does not require final hydrolysis.
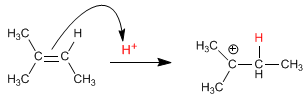
The mechanism occurs with the formation of a carbocation after adding the proton to the double bond. The hydration of alkenes is Markovnikov, that is, the proton adds to the less substituted carbon of the alkene (carbon with more hydrogens).
Stage 1. Attack of the alkene on the proton (electrophilic addition)
Stage 2. Nucleophilic attack of water on the formed carbocation
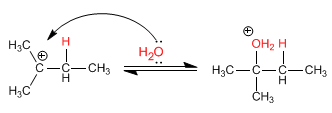
Stage 3. Deprotonation of alcohol. The water acts as a base.
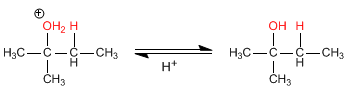
By Le Châtelier's principle, as the concentration of a reactant increases, the equilibrium shifts towards the final product. To increase the yield of this reaction, excess water can be added, causing a displacement of the equilibrium towards the final alcohol.