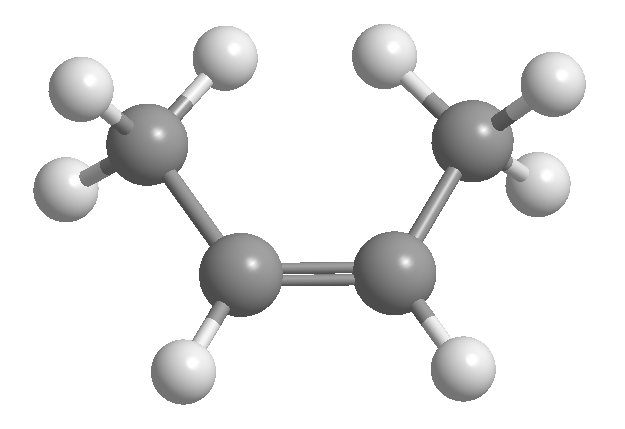
cis-trans or geometric isomerism is due to restricted rotation around a carbon-carbon bond. This restriction may be due to the presence of double bonds or cycles. Thus, 2-butene can exist in the form of two isomers, called cis and trans. The isomer with the hydrogens on the same side is called cis, and the one with the opposite sides is called trans.

[1] cis-2-Butene
[2] trans-2-Butene
Cyclic compounds, due to their rigidity, also exhibit geometric isomerism. Thus, 1,2-dimethylcyclohexane can exist as two isomers. The one with the hydrogens on the same side is called the cis isomer and the one with the hydrogens on the opposite sides is called the trans. 
[3] cis-1,2-Dimethylcyclohexane
[4] trans-1,2-Dimethylcyclohexane

[5] trans-2-Hexene
[6] cis-2-Hexene
![]() Note that the cis/trans notation is written in lowercase italics. In handwriting, italics are replaced by underlining.
Note that the cis/trans notation is written in lowercase italics. In handwriting, italics are replaced by underlining.