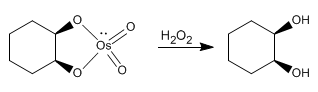
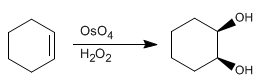
The dihydroxylation of an alkene consists of adding an -OH group to each carbon to form vicinal diols. This reaction can be carried out with osmium tetroxide in hydrogen peroxide, or with potassium permanganate in water.
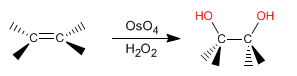
The product of this reaction is a vicinal diol WITHOUT
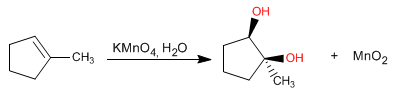
As the alkene is flat with two faces equally accessible to the reagent, a racemic mixture is obtained as the final product. Approaching the permanganate from the top face forms one enantiomer, while approximation from the bottom forms the other.
The reaction mechanism consists in the formation of a cycle, which is broken at a later stage, leaving the diol free.
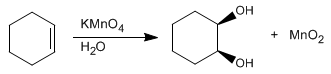
Stage 1. Condensation of the alkene with the permanganate
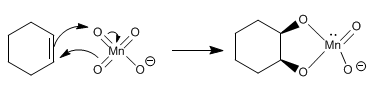
Stage 2 . Breaking the cycle releases the diol
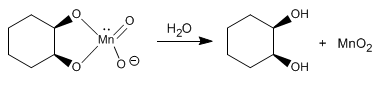
The osmium tetroxide mechanism follows analogous steps
Stage 1. Condensation of the alkene with osmium tetroxide
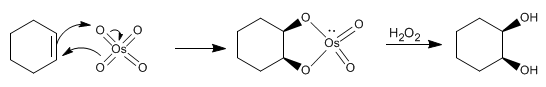
Stage 2. Oxidation stage that releases the diol