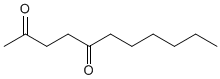
ALDEHYDE AND KETONE PROBLEMS
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 55497
Name the following aldehydes and ketones: 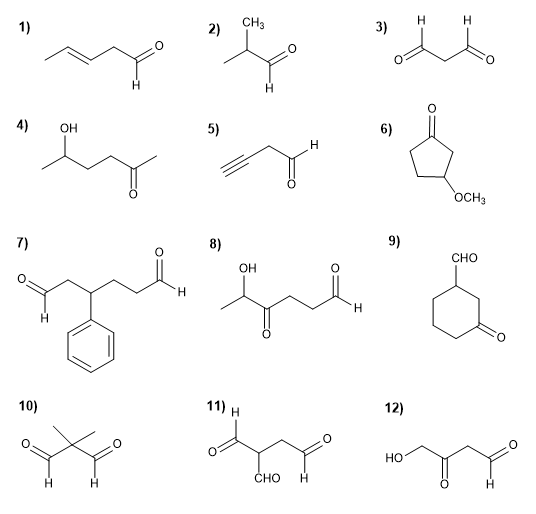
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 40148
1) Common names and structural formulas of some carbonyl compounds are given below. Indicate the corresponding name according to IUPAC. 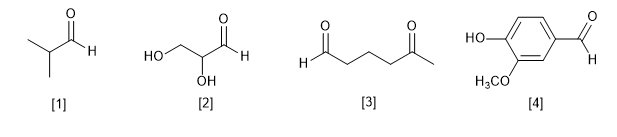
[1] Isobutyraldehyde
[2] Glyceraldehyde
[3] Glutaraldehyde
[4] Vanillin
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 62099
Draw the structure of the following aldehydes and ketones:
1) Ethanal (acetaldehyde) |
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 148628
Draw the structure of the acetal that forms when benzaldehyde is heated with 1,2-ethanediol in an acid medium. Write a detailed mechanism that justifies your formation. Describe step by step the hydrolysis of this acetal in an aqueous acid medium.
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 16090
When formaldehyde is dissolved in 17O-labeled water, it is observed that after a few hours both the formaldehyde hydrate and the formaldehyde have incorporated the 17O-isotope. Suggest a reasonable explanation for this fact.
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 16426
Suggest a reasonable mechanism for one of the following reactions: 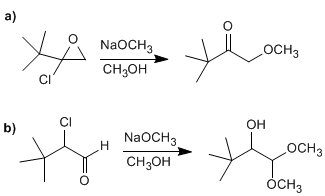
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 14678
Using ethanol as the source of all the carbon atoms and the reagents you need, describe an efficient synthesis of each of the following substances: 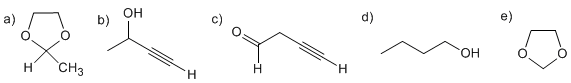
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 13427
Using the necessary reagents, indicate the steps that allow the following transformation to be carried out: 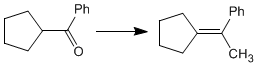
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 15244
From 5-hexenal and o-bromotoluene obtain the following product. 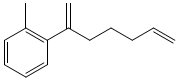
Additional organic and inorganic reagents may be necessary.
- Details
- Germán Fernández
- ALDEHYDE AND KETONE PROBLEMS
- Hits: 95968
Obtain from 3-chloro-2-methylbenzaldehyde and the necessary reagents 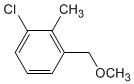
the following compound: