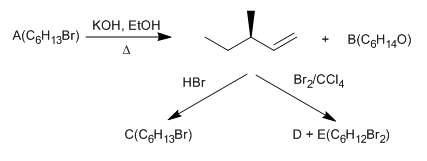
PROBLEMS REACTIONS OF ALKENES
- Details
- Germán Fernández
- PROBLEMS REACTIONS OF ALKENES
- Hits: 27813
Each of the following carbocations can evolve into a more stable one. Write the structure of the new carbocations.
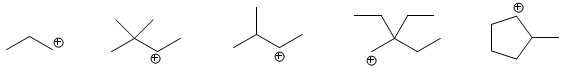
- Details
- Germán Fernández
- PROBLEMS REACTIONS OF ALKENES
- Hits: 25315
Write the reaction products of 2-methyl-1-pentene with each of the following reagents: (a) H2 , PtO2 (b) D2 , Pd-C; (c) 1. BH3 , 2. H2O2 /NaOH; (d) HCl; (e) HBr; (f) HBr, ROOR; (g) HI, ROOR; (h) H2SO4 , H2O; (i) Cl2 ; (j) ICl; (k) Br2 , EtOH; (l) 1. Hg(OAc)2 , H2O, 2. NaBH4 ; (m) MCPBA; (n) OsO4 (cat.), H2O2 ; (o) 1. O3 , 2. Zn, AcOH; (p) CH3SH, ROOR; (q) HCBr3 , ROOR; (r) H2SO4 (cat.)
a) H2 , PtO2 :
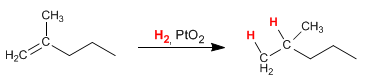
- Details
- Germán Fernández
- PROBLEMS REACTIONS OF ALKENES
- Hits: 20789
Write the mechanism that explains the following transformation:
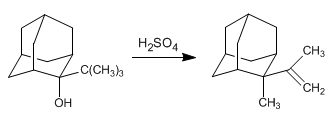
- Details
- Germán Fernández
- PROBLEMS REACTIONS OF ALKENES
- Hits: 31110
Suggest a suitable sequence of reactions to prepare each of the following compounds from the indicated starting material. You can use any organic or inorganic reagent that you consider necessary.
- Details
- Germán Fernández
- PROBLEMS REACTIONS OF ALKENES
- Hits: 15613
Compound A undergoes the catalytic hydrogenation reaction much faster than compound B. Why?
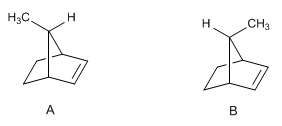
- Details
- Germán Fernández
- PROBLEMS REACTIONS OF ALKENES
- Hits: 16620
East Indian sandalwood oil contains a hydrocarbon called santene (C 9 H 14 ). Ozonolysis of the santene followed by hydrolysis forms compound A below. What is the structure of santeno?
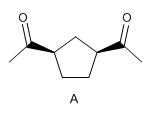
- Details
- Germán Fernández
- PROBLEMS REACTIONS OF ALKENES
- Hits: 18041
A single epoxide was isolated in 79-84% yield in the following reaction. Is this epoxy A or B? Reason the answer.
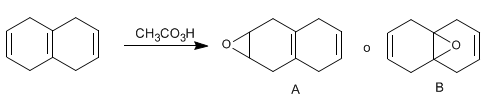
- Details
- Germán Fernández
- PROBLEMS REACTIONS OF ALKENES
- Hits: 16098
Complete the following sequence of reactions