SUBSTITUTION REACTIONS (SN1)
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN1)
- Hits: 57252
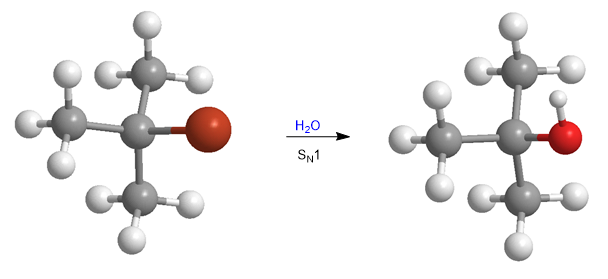
The steric hindrances make the tertiary substrates practically inert towards the SN2 mechanism. However, it is observed that these substrates react at an important rate with water, following first order kinetics.
This experimental observation implies proposing a new mechanism, called SN1, which occurs with tertiary substrates and poor nucleophiles, facts that are impossible to explain using the SN2 mechanism.
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN1)
- Hits: 72111
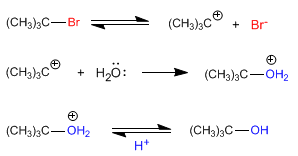
The SN1 has a staged mechanism. In the first step, the substrate is ionized by loss of the leaving group, without the nucleophile acting, forming a carbocation. In the second step, the nucleophile attacks the carbocation formed, obtaining the final product. ![]()
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN1)
- Hits: 51628
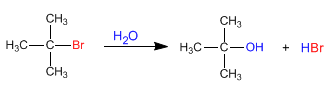
Let the overall reaction be:
![]()
The mechanism occurs in three steps
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN1)
- Hits: 47327
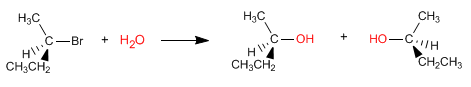
The SN1 reaction proceeds through a planar carbocation, which is attacked by the nucleophile on both sides, giving rise to a mixture of stereoisomers.
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN1)
- Hits: 44717
The SN1 mechanism, analogous to the SN2 mechanism, requires good leaving groups.
TsO- > I- > Br- > H2O > Cl-
Water does not react with 2-Fluoropropane since fluorine is a bad leaving group, but it does with 2-Bromopropane.
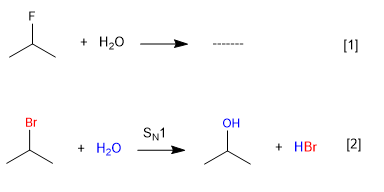
[1] Reaction does not take place (Fluorine bad leaving group)
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN1)
- Hits: 38691
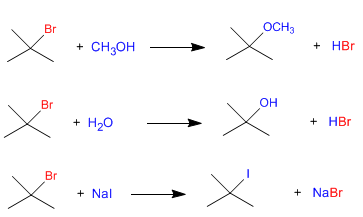
Since the slow step of unimolecular nucleophilic substitution (SN1) is dissociation of the substrate, and the nucleophile acts in the second step, the rate of the reaction does not depend on the nucleophile. The following three reactions proceed at the same speed since they start from the same substrate.
Obviously, it is necessary that other factors, such as solvent, be the same in the three reactions
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN1)
- Hits: 74031
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN1)
- Hits: 49703