SUBSTITUTION REACTIONS (SN2)
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN2)
- Hits: 57139
In nucleophilic substitution reactions (SN2), one group, called the leaving group, is exchanged for another, called the nucleophile. 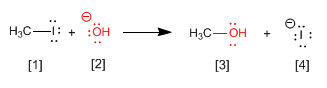
[1] Substrate - Species containing the leaving group
[2] Nucleophile - Lewis base capable of attacking atoms with positive polarity (or charge)
[3] Reaction product
[4] Leaving group - Species that leaves the substrate, being replaced by the nucleophile
Read more: General Characteristics of Bimolecular Nucleophilic Substitution (SN2)
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN2)
- Hits: 76267
The SN2 (bimolecular nucleophilic substitution) is a concerted reaction, that is, it occurs in a single step.
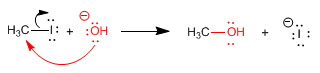
Read more: Mechanism - Bimolecular nucleophilic substitution - SN2
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN2)
- Hits: 53648
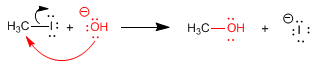
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN2)
- Hits: 51488
Nucleophile attack on the carbon containing the leaving group can occur in two different ways. In the first case, the nucleophile can approach the substrate from the side on which the leaving group is located. This approach is called frontal attack, in which the nucleophile takes the place of the leaving group, producing retention in the configuration .
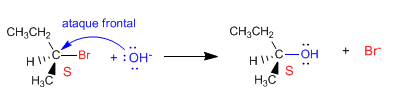
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN2)
- Hits: 68195
The leaving group has the mission of leaving the substrate at the same time that the nucleophile attacks. The best leaving groups are the less basic species, since they bond weakly to carbon. In the periodic table the best leaving groups are to the right and bottom.
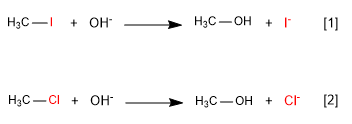
[1] Fast
[2] Slow
Read more: The leaving group in the nucleophilic substitution - SN2
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN2)
- Hits: 66757
Nucleophiles are Lewis bases that attack a carbon, displacing the leaving group. Ionic nucleophiles are common, but there are also numerous examples of neutral nucleophiles. The general characteristic of all nucleophiles is the presence of lone pairs on the attacking atom.
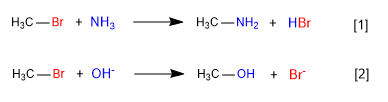
[1] Slow
[2] Fast
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN2)
- Hits: 50011
There is a significant speed difference between the substrates; methyl bromide, ethyl bromide, isopropyl bromide and tert-butyl bromide when reacted with a nucleophile under the same conditions.
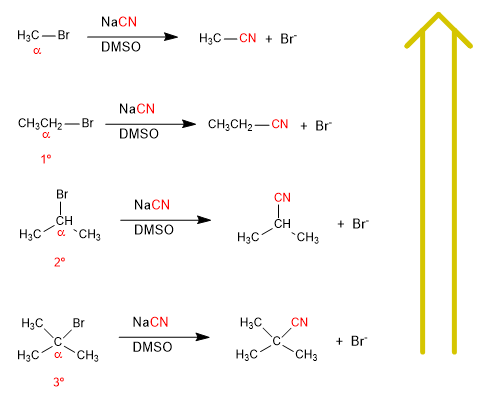
As you move up the list of reactions, the speed increases.
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN2)
- Hits: 36125
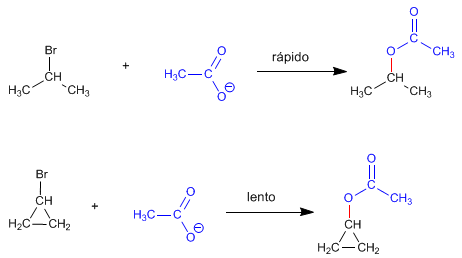
Stressed cyclic substrates react more slowly via the SN2 mechanism than acyclic substrates. Thus, isopropyl bromide reacts faster with the acetate ion than does cyclopropyl bromide.
- Details
- Germán Fernández
- SUBSTITUTION REACTIONS (SN2)
- Hits: 50446
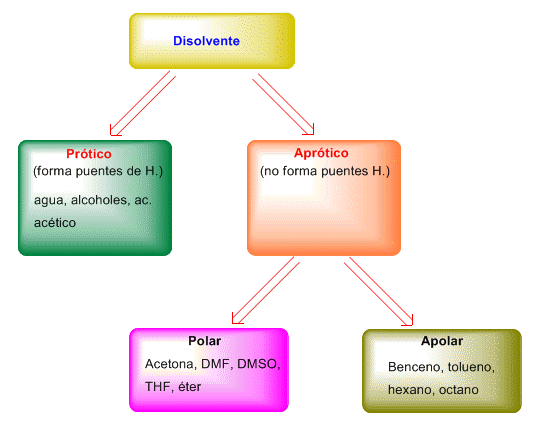
Solvents can be classified according to the following scheme.