THEORY OF CYCLOALKANES
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 1991
Cycloalkanes nomenclature 
Cycloalkanes are named with the prefix cyclo- followed by the name of the alkane with the same number of carbons. Cycloalkanes exhibit cis/trans isomerism. When the substituents are on the same face of the molecule, they are said to be cis; when they meet on opposite sides, they are said to be trans.
Physical properties
They have higher melting and boiling points than the corresponding alkanes of the same number of carbons. The rigidity of the ring allows a greater number of intermolecular interactions, which it is necessary to break through the input of energy, to pass the molecules to the gas phase.
Annular tension
Small size cycloalkanes (cyclopropane, cyclobutane) present significant stress due to bond angles and eclipsing. Larger cycloalkanes such as cyclopentane and cyclohexane are nearly stress-free.
Conformational isomers in cyclohexane
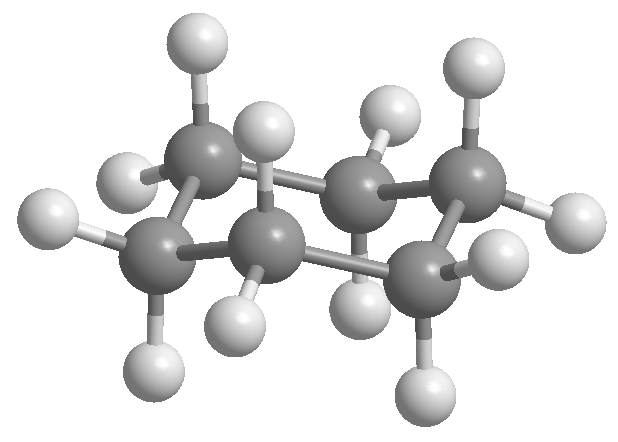
Cyclohexane is arranged in the form of a chair to avoid eclipsing between hydrogens. The chair form of cyclohexane contains two types of hydrogens; the axial ones that are located perpendicular to the plane of the molecule and the equatorial ones placed in the same plane.
Equatorial-axial equilibrium in substituted cyclohexanes
Cyclohexane presents a conformational equilibrium that interconverts equatorial hydrogens to axial ones and vice versa. When a cyclohexane is substituted, the conformation that places the most groups in the equatorial position is the most stable, finding the conformational equilibrium shifted towards said conformation.
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 163296
Cycloalkanes are alkanes that have the chain ends joined together, forming a cycle. They have two fewer hydrogens than the alkane from which they are derived, which is why their molecular formula is CnH2n . They are named using the prefix cyclo followed by the name of the alkane.
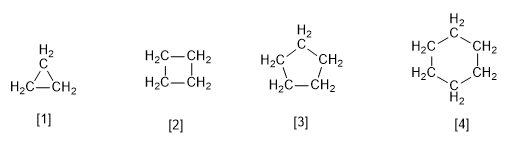
[1] Cyclopropane
[2] Cyclobutane
[3] Cyclopentane
[4] Cyclohexane
It is common to represent molecules indicating only their skeleton. Each vertex represents a carbon bonded to two hydrogens. 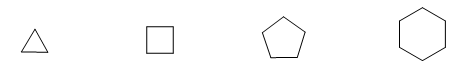
The IUPAC rules for naming cycloalkanes are very similar to those studied for alkanes.
Rule 1.- In cycloalkanes with a single substituent, the cycle is taken as the main chain of the molecule. Cycle numbering is unnecessary.
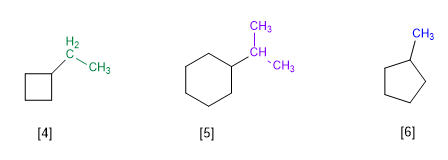
[4] Ethylcyclobutane
[5] Isopropylcyclohexane
[5] Methylcyclopentane
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 81573
In nature, compounds with cycles of five and six links are very abundant. However, the three- and four-membered cycles appear very rarely in natural products.
Stability in cycloalkanes
These experimental facts suggest the greater stability of the cycles of five or six members with respect to those of three or four.
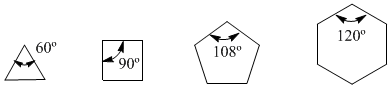
In the year 1885, the German chemist Adolf von Baeyer proposed that the instability of small cycles was due to the tension of the bond angles. The sp3 carbons have natural bond angles of 109.5º, in cyclopropane these angles are 60º, which is a deviation of 49.5º. This deflection translates into stress, which causes instability in the molecule.
Cyclobutane is more stable since its bond angles are 90º and the deflection is only 19.5º. Baeyer applied this reasoning to the other cycloalkanes and predicted that cyclopentane should be more stable than cyclohexane.
Angle strain
Observe the bond angles of the different cycloalkanes:
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 70025
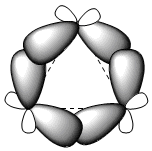 The three carbon atoms of cyclopropane lie in the same plane, unlike the other cycloalkanes which are not planar. Cyclopropane is characterized by a large angular strain due to bond angles well below 109.5°. The enormous strain to which the cycle is subjected causes the carbon-carbon bonds to bend outwards, giving rise to very characteristic bonds called "banana" bonds.
The three carbon atoms of cyclopropane lie in the same plane, unlike the other cycloalkanes which are not planar. Cyclopropane is characterized by a large angular strain due to bond angles well below 109.5°. The enormous strain to which the cycle is subjected causes the carbon-carbon bonds to bend outwards, giving rise to very characteristic bonds called "banana" bonds.
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 64897
Cyclobutane is not flat, one of its carbons comes out about 25º from the plane formed by the other three carbons. This arrangement increases the angular tension, but the hydrogen-hydrogen eclipsing that the molecule presents in its planar form is considerably reduced.
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 51740
If cyclopentane were planar, it would be practically free of angle strain. But it would present five eclipsed hydrogens for each face, which would destabilize the molecule. The most favorable arrangement of cyclopentane is the envelope form. In this arrangement the sum of angular stresses and eclipsing is minimal.
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 73859
 Cycles of six are the most abundant in nature. There are many compounds with biological activity whose base are cycles of six condensed atoms (cholesterol).
Cycles of six are the most abundant in nature. There are many compounds with biological activity whose base are cycles of six condensed atoms (cholesterol).
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 73419
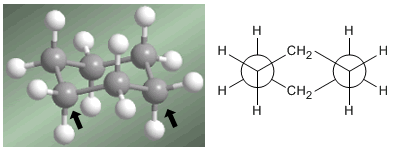
Professor Odd Hassel of the University of Oslo established that the most stable conformation of cyclohexane is the chair form. With bond angles of 111º the chair is almost free of angular tension. Also, all the links are staggered as can be seen in the Newman projection.
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 4444
Cyclohexane presents a conformational equilibrium in which the axial hydrogens, in red, move to the equatorial position. The equatorial hydrogens, in green, turn to the axial position.
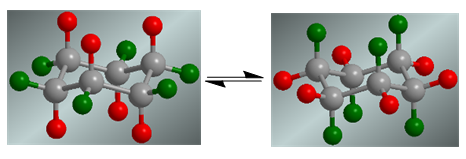
In the absence of substituents, both conformations have the same probability of existing and the coformational equilibrium is not shifted.
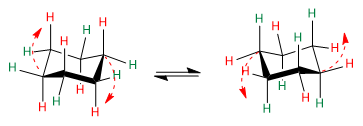
Observe the red arrows to understand how the transition from one conformation to another takes place.
- Details
- Germán Fernández
- THEORY OF CYCLOALKANES
- Hits: 7787
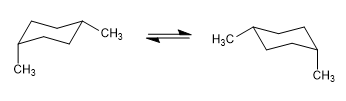
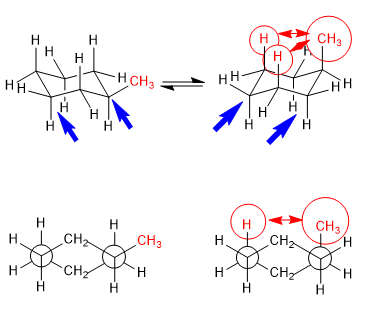
In the following model it can be seen that in the equatorial conformer the methyl group is far from the rest of the groups. On the contrary, in the axial conformer said methyl group is facing the axial hydrogens that are located in position 3 with respect to it. This spatial proximity causes a steric repulsion, called the 1,3-diaxial interaction.
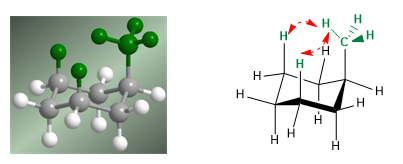
The 1,3-diaxial interaction causes a shift of the conformational equilibrium to the left. The conformation on the right has a high energy due to the repulsion between methyl and axial hydrogens.
Equilibrium in trans-1,4-dimethylcyclohexane
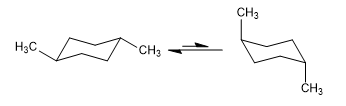
The 1,3-diaxial interactions make the substituents tend to be located in equatorial positions. Thus, in trans-1,4-Dimethylcyclohexane the conformation with the two methyl groups in equatorial is more stable than the chair with axial methyls, this produces a displacement of the conformational equilibrium to the left.
Equilibrium in cis-1,4-dimethylcyclohexane