THEORY OF ALCOHOLS
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 176341
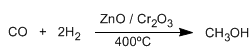
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 180259
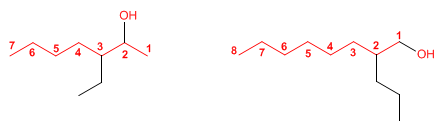
Rule 1. The longest chain that contains the -OH group is chosen as the main chain.

- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 117012
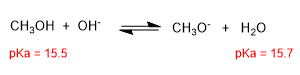
Alcohols are amphoteric (amphiprotic) species, they can act as acids or bases. In aqueous solution an equilibrium is established between alcohol, water and their conjugate bases.
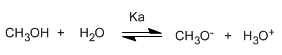
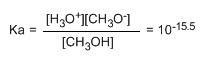
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 79100
Alcohols can be obtained from haloalkanes by SN2 and SN1 reactions
Synthesis of alcohols using SN2
Primary haloalkanes react with sodium hydroxide to form alcohols. Secondary and tertiary haloalkanes eliminate to form alkenes.
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 92079
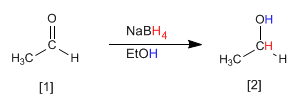
Both sodium borohydride $(NaBH_4)$ and lithium aluminum hydride $(LiAlH_4)$ reduce aldehydes and ketones to alcohols.
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 60585
Another method of preparing alcohols is the reduction of aldehydes or ketones to alcohols. The simplest method is the hydrogenation of the carbon-oxygen double bond, using hydrogen in the presence of a platinum, palladium, nickel, or ruthenium catalyst.
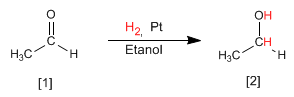
Read more: Synthesis of Alcohols by Hydrogenation of Carbonyls
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 57810
Alcohols can be obtained by opening epoxides (oxacyclopropanes). This opening can be done using organometallic reagents or the lithium aluminum reductant.
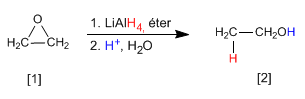
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 79705
A synthesis method for alcohols, already studied in the section on alkenes, consists in hydrating the alkene. The addition of -OH can be on the more substituted carbon of the alkene (Markovnikov), or on the less substituted carbon (anti-Markovnikov).
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 56838
Carboxylic acids and esters are reduced to alcohols with lithium aluminum hydride. Milder reducers such as sodium borohydride are unable to reduce these compounds.
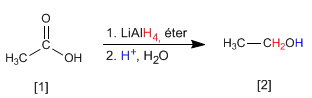
Read more: Synthesis of alcohols by reduction of acids and esters
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 55931
Primary and secondary alcohols can be converted to haloalkanes with reagents such as: phosphorus tribromide, phosphorus trichloride, thionyl chloride, and phosphorus pentachloride.
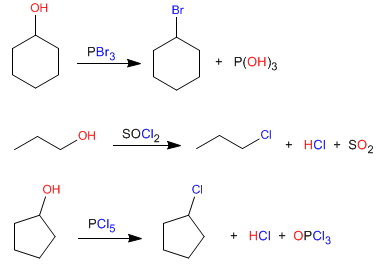
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 170169
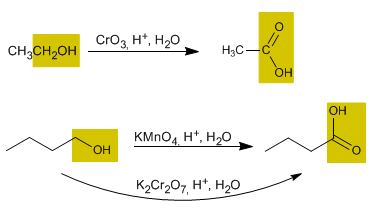
The oxidation of alcohols forms carbonyl compounds. Aldehydes are obtained by oxidizing primary alcohols, while oxidizing secondary alcohols forms ketones.
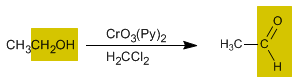
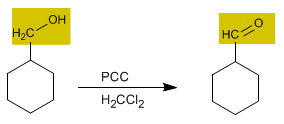
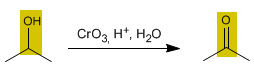
- Details
- Germán Fernández
- THEORY OF ALCOHOLS
- Hits: 90439
Alkoxides are the bases of alcohols, they are obtained by reacting alcohol with a strong base.