ACID HALIDE THEORY
- Details
- Germán Fernández
- ACID HALIDE THEORY
- Hits: 32449
IUPAC names alkanoyl halides by replacing the -oic acid ending with an equal number of carbons per -oil . In addition, the word acid is replaced by the corresponding halogen, named as salt.
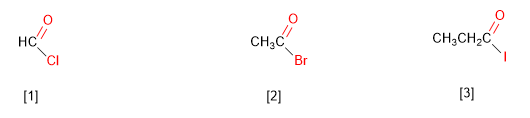
[1] Methanoyl chloride
[2] Ethanoyl bromide
[3] Propanoyl iodide
- Details
- Germán Fernández
- ACID HALIDE THEORY
- Hits: 15746
Alkanoyl halides react with water at room temperature to form carboxylic acids.
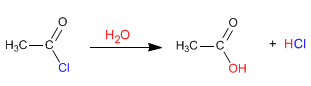
- Details
- Germán Fernández
- ACID HALIDE THEORY
- Hits: 12954
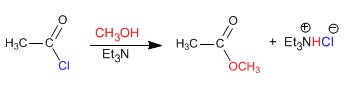
The reaction of alkanoyl halides with alcohols produces esters. The equilibria of this reaction are favored by eliminating the hydrochloric acid with a base (tertiary amine, pyridine)
- Details
- Germán Fernández
- ACID HALIDE THEORY
- Hits: 18984
Amines and ammonia react with alkanoyl halides to form amides. The reaction is favored with an excess of amine, in order to eliminate the hydrochloric acid released in the reaction.
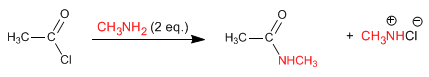
Read more: Reaction of alkanoyl halides with amines and ammonia
- Details
- Germán Fernández
- ACID HALIDE THEORY
- Hits: 10705
Magnesium organometallics at -78°C react with alkanoyl halides to form ketones. It is necessary to work at a low temperature to avoid adding a second equivalent of organometallic, in which case the product obtained would be an alcohol.

Read more: Reaction of Alkanoyl Halides with Organometallics
- Details
- Germán Fernández
- ACID HALIDE THEORY
- Hits: 17790
Alkanoyl halides are reduced to alcohols with the lithium aluminum reductant
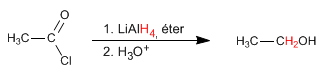
- Details
- Germán Fernández
- ACID HALIDE THEORY
- Hits: 19407
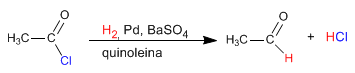
The reduction of alkanoyl halides can be stopped at the aldehyde using modified hydrides. From lithium aluminum hydride lithium tri(tert-butoxy)aluminum hydride can be prepared. This modified hydride allows reduction of acid halides to aldehydes.
- Details
- Germán Fernández
- ACID HALIDE THEORY
- Hits: 18767