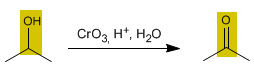The oxidation of alcohols forms carbonyl compounds. Aldehydes are obtained by oxidizing primary alcohols, while oxidizing secondary alcohols forms ketones.
1. Oxidation of primary alcohols to aldehydes
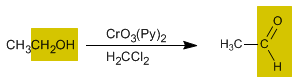
Chromium trioxide with pyridine in dichloromethane allows the isolation of aldehydes with good yield from primary alcohols.
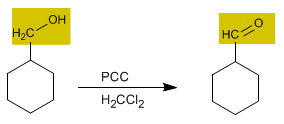
Chromium trioxide with pyridine and hydrochloric acid in dichloromethane is known as PCC (pyridinium chlorochromate). This reagent also converts primary alcohols to aldehydes.
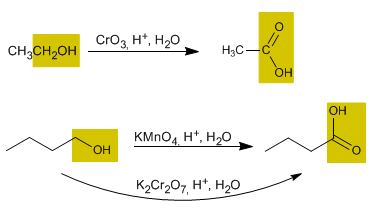
2. Oxidation of primary alcohols to carboxylic acids
Chromium trioxide in an aqueous acidic medium (Jones' reagent), potassium permanganate, and potassium dichromate oxidize primary alcohols to carboxylic acids.
3. Oxidation of secondary alcohols to ketones
Oxidants convert secondary alcohols to ketones. Overoxidation to carboxylic acid is not possible.