Name the following alkenes: 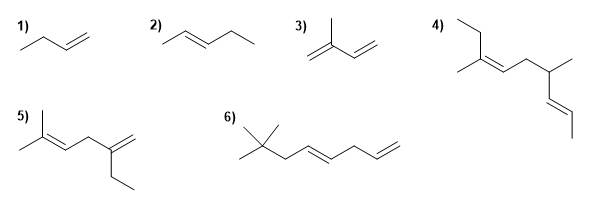
SOLUTION:
Molecule 1.
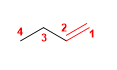
1. Main chain: 4 carbons with double bond (butene)
2. Numbering: lowest locant to the double bond.
3. Substituents: no
4. Name: But-1-ene
Molecule 2.
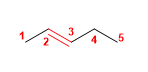
1. Main chain: 5 carbons with double bond (pentene)
2. Numbering: lowest locant to the double bond.
3. Substituents: no
4. Name: Pent-2-ene
Molecule 3.
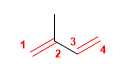
1. Main chain: 4 carbons with two double bonds (butadiene)
2. Numbering: double bonds at the same distance from the ends, we number so that methyl takes the lowest locant.
3. Substituents: methyl in position 2 .
4. Name: 2-Methylbuta-1,3-diene
Molecule 4.
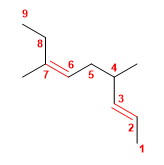
1. Main chain: 9 carbons with two double bonds ( nonadiene )
2. Numbering: lowest locant to the double bonds ( 2,6 ).
3. Substituents: methyls in position 4,7 .
4. Name: 4,7-Dimethylnon-2,6-diene
![]() The longest chain containing the greatest number of double bonds is chosen as the main chain.
The longest chain containing the greatest number of double bonds is chosen as the main chain.
Molecule 5.
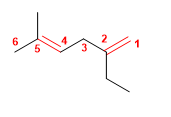
1. Main chain: 6 carbons with two double bonds (hexadiene). Note that it is not the longest.
2. Numbering: lowest locant to the double bonds ( 1,4 ).
3. Substituents: 2 -position ethyl and 5 -position methyl.
4. Name: 2-Ethyl-5-methylhexa-1,4-diene
Molecule 6.
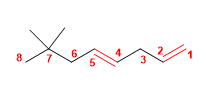
1. Main chain: 8 carbons with two double bonds (octadiene)
2. Numbering: lowest locant to the double bonds ( 1,4 ).
3. Substituents: methyls in position 7,7 .
4. Name: 7,7-Dimethylocta-1,4-diene ![]() The main chain is numbered so that the double bonds take the minor locants.
The main chain is numbered so that the double bonds take the minor locants.