Alkenes, in the presence of concentrated sulfuric acid, condense to form chains called polymers. Let's see an example with 2-Methylpropene
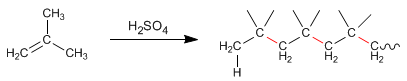
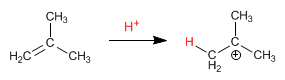
Step 1. Protonation of the double bond to form the tert-butyl cation
Stage 2. Nucleophilic attack of the alkene to the formed carbocation.
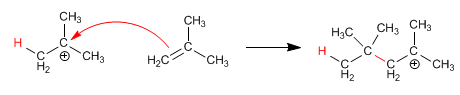
Stage 3. The cation formed in the previous stage is again attacked by another alkene molecule, forming the polymer.
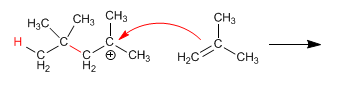
Because this type of polymerization occurs with the formation of carbocations, it is called cationic polymerization.
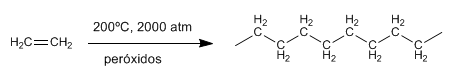
Ethene cannot polymerize via carbocation (it forms unstable carbocations), but polyethylene can be made by heating ethylene at high pressures and in the presence of peroxides. In this case the polymerization follows a radical mechanism and is called "free radical" polymerization .