Alkanes are sp3 hybridized compounds on all carbons. The four substituents that start from each carbon are arranged towards the vertices of a tetrahedron.
Bond distances and angles are shown on the following models. 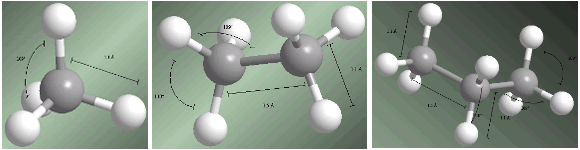
The smaller alkanes, methane, ethane, propane, and butane, are gases at room temperature. Linear alkanes from C5H12 to C17H36 are liquids. alkanes of  higher number of carbons are solid at room temperature.
higher number of carbons are solid at room temperature.
The melting and boiling points of alkanes increase with the number of carbons in the molecule. It is also observed that branched alkanes have a lower boiling point than their linear isomers.
The following graph shows the boiling points of linear alkanes (in black) and those corresponding to their 2-methylalkanes isomers (in blue). 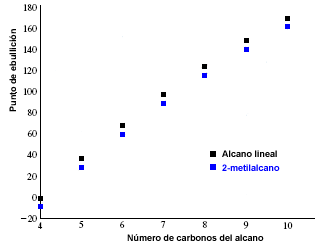
In the liquid phase there are forces of attraction between molecules that hold them together. To pass the substance to the gas phase, it is necessary to overcome these intermolecular forces through the input of energy. 
In neutral molecules, such as alkanes, the attractive forces are due to van der Waals interactions that can be of three types: dipole-dipole, dipole-induced dipole and induced dipole-induced dipole interactions.
The formation of induced dipoles that produce the attraction between neutral molecules can be seen in the following scheme: 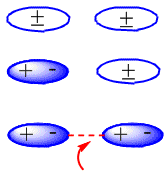
Let us consider the pentane isomers, as an example of the decrease in boiling point, when going from linear to branched alkanes.
Pentane has a large surface area that allows for a large number of induced dipole – induced dipole interactions. 2-Methylbutane is more compact and has a lower surface area, fewer intermolecular interactions, and a lower boiling point.