ALKENE THEORY
- Details
- Germán Fernández
- ALKENE THEORY
- Hits: 74693
 Alkenes are hydrocarbons that contain carbon-carbon double bonds. The word olefin is frequently used as a synonym.
Alkenes are hydrocarbons that contain carbon-carbon double bonds. The word olefin is frequently used as a synonym.
Alkenes abound in nature. Ethene is a compound that controls plant growth, seed germination and fruit ripening.
- Details
- Germán Fernández
- ALKENE THEORY
- Hits: 184128
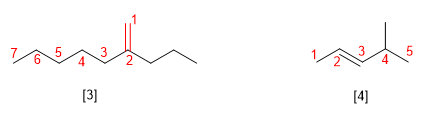
Alkenes are named by replacing the -ane ending of the corresponding alkane with -ene. The simplest alkenes are ethene and propene, also called ethylene and propylene at the industrial level.

- Details
- Germán Fernández
- ALKENE THEORY
- Hits: 119126
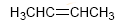 Each ring or cycle of a molecule implies the loss of two hydrogens with respect to an alkane of formula CnH2n+2 . The degree of unsaturation is the number of cycles and double bonds present in a molecule.
Each ring or cycle of a molecule implies the loss of two hydrogens with respect to an alkane of formula CnH2n+2 . The degree of unsaturation is the number of cycles and double bonds present in a molecule.
- Details
- Germán Fernández
- ALKENE THEORY
- Hits: 75631
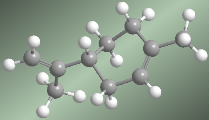
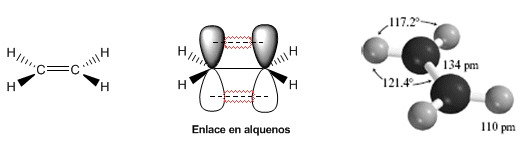
The following models show the structure, distances, and bond angles of ethene. Each of the carbons in the molecule is $sp^2$ hybridized. Its geometry is flat, with bond angles close to 120º.
- Details
- Germán Fernández
- ALKENE THEORY
- Hits: 129776
Alkenes have melting and boiling points close to the corresponding alkanes.
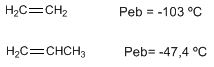
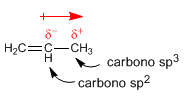
Dipole moment in alkenes. In carbon $sp^2$ the electrons in the s orbital are closer to the nucleus and are strongly attracted to it, so that a carbon $sp^2$ has a tendency to attract electrons towards itself, which generates dipole moments.
- Details
- Germán Fernández
- ALKENE THEORY
- Hits: 74179
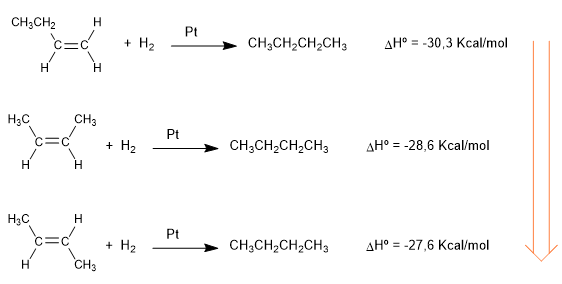
The heats released in the following hydrogenation reactions give us an idea about the different stability of alkenes.
- Details
- Germán Fernández
- ALKENE THEORY
- Hits: 67333
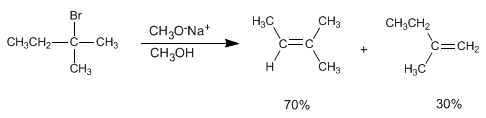
Alkenes can be prepared from haloalkanes and alkyl sulfonates by bimolecular elimination (E2). In the following example 2-bromo-2-methylbutane reacts with sodium methoxide to form a mixture of 2-methyl-2-butene and 2-methyl-1-butene.
- Details
- Germán Fernández
- ALKENE THEORY
- Hits: 123783
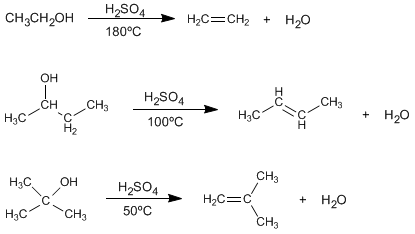
The treatment of alcohols with mineral acids at high temperatures causes the loss of water, which occurs through E1 or E2 mechanisms.