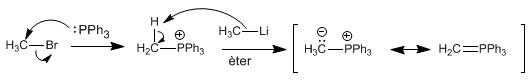The Wittig reaction employs phosphorus ylides [2] to transform aldehydes and ketones[1] in alkenes [3]. Triphenylphosphine oxide [4] is obtained as a by-product . 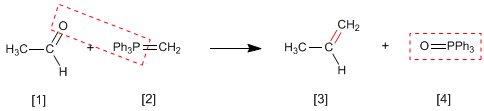
In the mechanism of the reaction, the ylide and the carbonyl combine to form an oxaphosphetane that breaks, leaving the final alkene free.
Stage 1. Ethanal and ylide combine to form phosphethane. 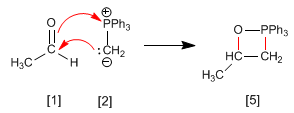
Stage 2. The phosphethane breaks down to form the alkene and triphenylphosphine oxide. 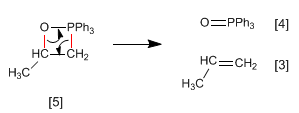
Example - Obtain by Wittig the 2-Methylbut-2-ene 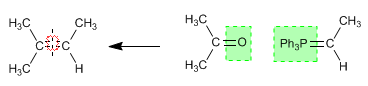
The alkene is broken by the double bond and the group circled in green is added to each carbon.
Phosphorus ylides are prepared by reaction of haloalkanes and triphenylphosphine, followed by strong base carbon deprotonation (lithium organometallics).