Hydrocarbons are compounds that contain only carbon and hydrogen. They are divided into two classes: aliphatic and aromatic hydrocarbons.
Aliphatic hydrocarbons include three classes of compounds: alkanes, alkenes, and alkynes. Alkanes are hydrocarbons that contain only carbon-carbon single bonds, alkenes contain carbon-carbon double bonds, and alkynes are hydrocarbons that contain one triple bond. 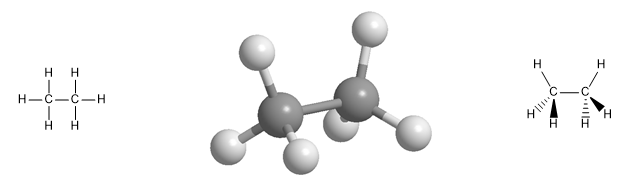
Molecular model of the ethane molecule. Ethane has a tetrahedral geometry, with carbon at the center of the tetrahedron and hydrogens oriented towards its vertices. Note the spatial representation with wedges and dashed lines .
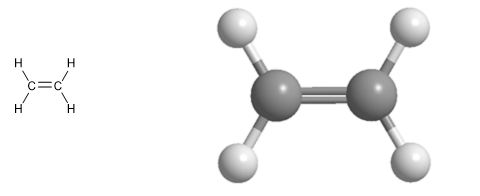
Molecular model of ethene. Ethene has a flat geometry, all its atoms are arranged in the same plane. Its main characteristic is the double bond that joins its carbons.
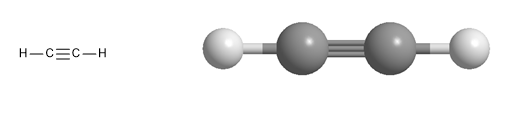
Molecular model of ethyne. Ethyne is a linear molecule, all its atoms are located on an axis. It is characterized by the triple bond that joins its carbons.
The second group is made up of aromatic hydrocarbons. The most important compound in this family is benzene. 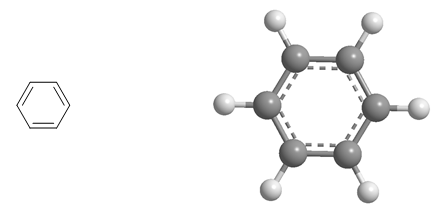
Molecular model of benzene. Benzene is a hydrocarbon, called aromatic, due to its great stability. The stability of benzene is due to delocalization of charge in the ring (resonance)
In short, hydrocarbons are molecules that only contain carbon and hydrogen in their structure. Obviously there are a large number of compounds that contain other additional atoms, such as oxygen (alcohols), nitrogen (amines), sulfur (thiols)......