Methane (CH 4 ), ethane (C 2 H 6 ) and propane (C 3 H 8 ) are alkanes with only one possible structure. However, there are two alkanes with the formula C 4 H 10 ; butane and 2-methylpropane. These alkanes with the same formula but different structures are called isomers.

[1] Butane
[2] 2-Methylpropane
n-Butane and isobutane have the same formula but differ in the way their atoms are joined -they are structural isomers-. Their different structure makes them have different properties, thus, they differ by about 20ºC in their melting point and about 10ºC in their boiling point.
There are three isomers with the formula C5H12 . The linear isomer is called n-pentane. The branched ones are isopentane (2-methylbutane) and neopentane (2,2-dimethylpropane).
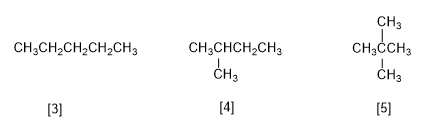
[3] Pentane
[4] 2-Methylbutane
[5] 2,2-Dimethylpropane
There are five constitutional isomers of formula C6H14 :
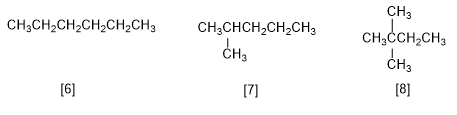
[6] Hexane
[7] 2-Methylpentane
[8] 2,2-Dimethylbutane
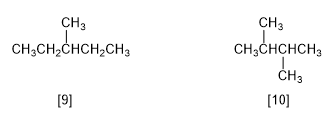
[9] 3-Methylpentane
[10] 2,3-Dimethylbutane
As the number of carbons increases, the number of isomers increases exponentially. There are more than 360,000 isomers with the formula C20H42 and more than 62 million with the formula C40H82 .