The leaving group has the mission of leaving the substrate at the same time that the nucleophile attacks. The best leaving groups are the less basic species, since they bond weakly to carbon. In the periodic table the best leaving groups are to the right and bottom.
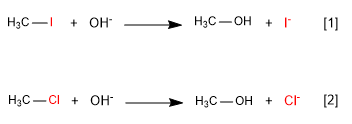
[1] Fast
[2] Slow
Iodide is a better leaving group than chloride because it is lower on the periodic table. Good leaving groups increase the rate of nucleophilic substitution.
There are leaving groups that act through oxygen, an atom much more basic than the halogens, taking advantage of resonance, which considerably decreases the basicity of the group. An example of this type is the tosylate ion.
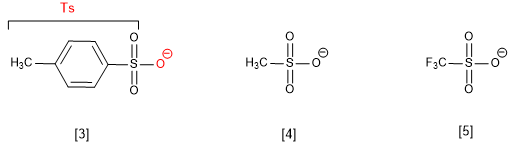
[3] Tosylate ion
[4] Mesylate ion
[5] Triflate ion
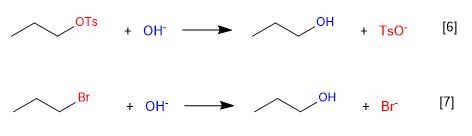
The reaction [6] is faster than that [7] since the tosylate is a better leaving group than the bromide.
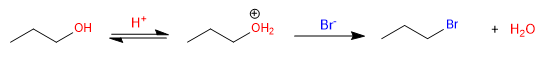
The -OH does not behave as a leaving group because it is very basic, but when it is protonated it becomes water, which does have aptitude as a leaving group.
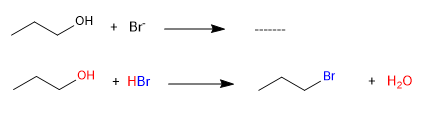
The first reaction does not take place because -OH does not behave as a leaving group. The second reaction occurs without problem thanks to the protonation of the -OH group in the acid medium, which transforms it into water, a good leaving group.
To finish I include a list with the best outgoing groups:
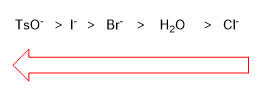
Best Outgoing Group
Example - Order the following substitution reactions according to rate
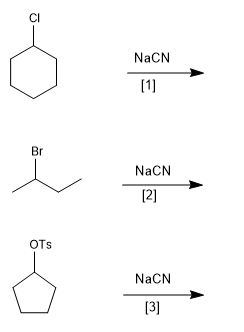
Solution: [3]>[2]>[1] . The tosylate is the best leaving group of the three, followed by the bromide and the chloride the worst.