THEORY OF CARBOXYLIC ACIDS
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 134511
IUPAC names carboxylic acids by replacing the ending -e of the alkane with the same number of carbons with -oic .
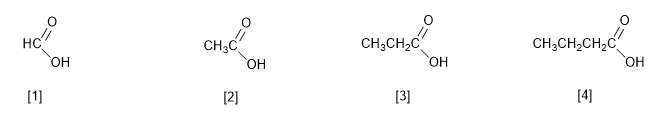
[1] Methanoic acid (Formic acid)
[2] Ethanoic acid (Acetic acid)
[3] Propanoic acid (Propionic acid)
[4] Butanoic acid (Butyric acid)
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 114864
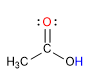
Carboxylic acids are molecules with trigonal planar geometry. They have acidic hydrogen in the hydroxyl group and behave like bases on carbonyl oxygen.
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 86269
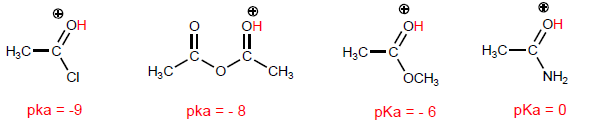
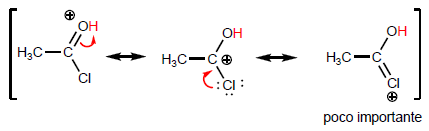
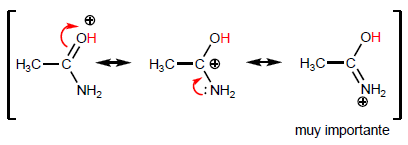
The most characteristic property of carboxylic acids is the acidity of the hydrogen located on the hydroxyl group. The pKa of this hydrogen ranges from 4 to 5 depending on the length of the carbon chain.
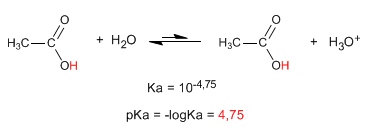
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 141278
Carboxylic acids can be prepared using the following methods:
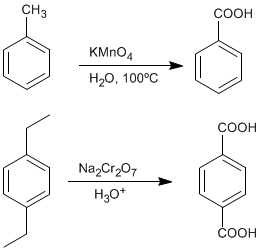
♦ Oxidation of alkylbenzenes: Carboxylic acids can be obtained from benzenes substituted with alkyl groups by oxidation with potassium permanganate or sodium dichromate.
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 62698
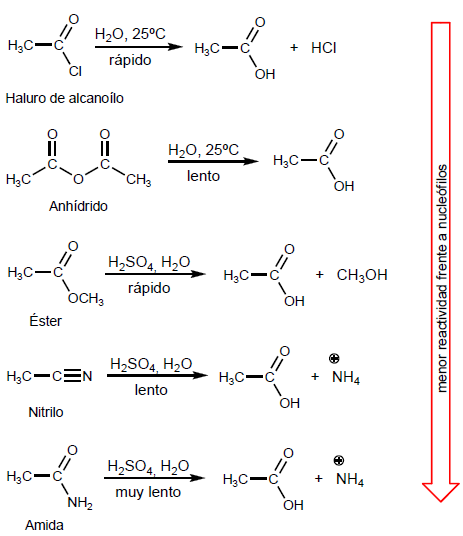
Alkanoyl halides are obtained by reacting carboxylic acids with PBr 3 . SOCl 2 can also be used.
Thus, ethanoic acid is transformed into ethanoyl bromide by reaction with phosphorous tribromide. Ethanoic acid reacts with thionyl chloride to form the compound
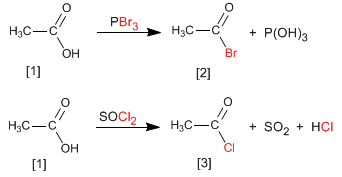
Read more: Synthesis of alkanoyl halides from carboxylic acids
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 73031
The anhydrides are obtained by condensation of carboxylic acids with loss of water. The reaction requires strong heating and a long reaction time.
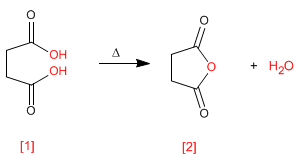
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 110618
Esters are obtained by reaction of carboxylic acids and alcohols in the presence of mineral acids. The reaction is carried out in excess of alcohol to shift the equilibria to the right. The presence of water is detrimental since it hydrolyzes the ester formed.
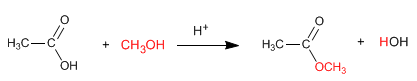
Read more: Synthesis of esters from carboxylic acids - Esterification
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 70254
Lactones are cyclic esters that are obtained by intramolecular esterification from molecules containing acid and alcohol groups. This cyclization forms 5- or 6-membered cycles.
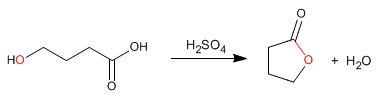
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 77206
Amides are formed by the reaction of carboxylic acids with ammonia, primary and secondary amines. The reaction is carried out under heating.
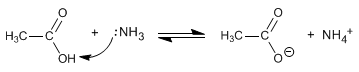
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 47943
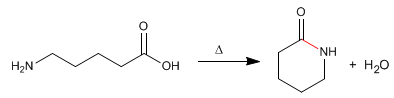
Lactams are cyclic amides formed from molecules that contain carboxylic and amine groups. The reaction is carried out by heating in the absence of acid.
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 45418
Carboxylic acids react with two equivalents of organolytic followed by aqueous hydrolysis to form ketones.
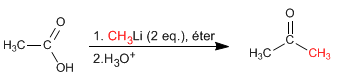
Read more: Reaction of carboxylic acids with organometallics
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 65515
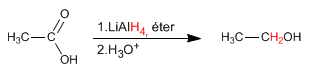
Lithium aluminum hydride reduces carboxylic acids to alcohols.
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 33857
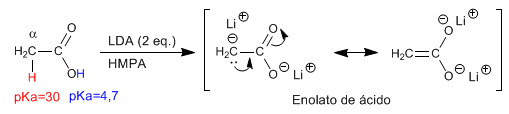
The a -hydrogens of carboxylic acids are acidic and can be removed using strong bases such as LDA.
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 45411
The Hell-Volhard-Zelinsky reaction makes it possible to halogenate the a-position of carboxylic acids. Phosphorus-catalyzed bromine is used as the reagent. Phosphorus in the presence of bromine generates phosphorus tribromide, which is actually the compound that acts as a catalyst.
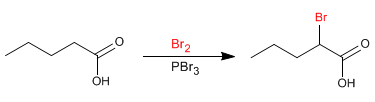
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 30771
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 34070
- Details
- Germán Fernández
- THEORY OF CARBOXYLIC ACIDS
- Hits: 21863