THEORY OF BENZENE
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 151072
Monosubstituted benzenes are named by ending the name of the substituent in benzene.
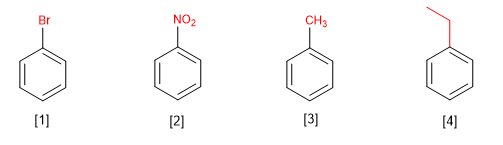
[1] Bromobenzene
[2] Nitrobenzene
[3] Methylbenzene (toluene)
[4] Ethylbenzene
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 86333
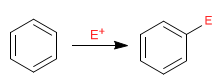
Benzene acts as a nucleophile, attacking a large and varied number of electrophiles.
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 124328
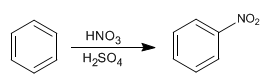
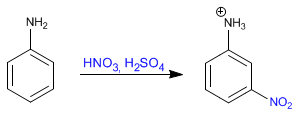
The benzene reacts with the nitric-sulfuric mixture adding nitro groups.
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 116191
The reaction of benzene with a solution of sulfur trioxide in sulfuric acid produces benzenesulfonic acids. 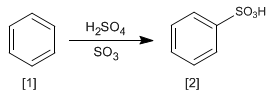
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 101564
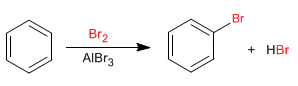
Benzene reacts with halogens in the presence of Lewis acids to form halogenated derivatives.
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 74248
Read more: Benzene - Protection and deprotection of the amino group
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 4459
The amino group is introduced into the aromatic ring by reduction of the nitro.
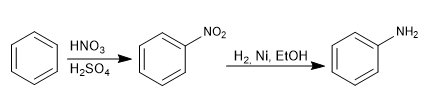
The reagents used in the reduction can be:
- Sn, HCl
- H2 , Ni, EtOH
- Fe, HCl
Read more: Reduction of nitro to amino and oxidation of amino to nitro
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 4133
The reversibility of sulfonation allows it to be used to protect activated positions of benzene. Let's see an example:
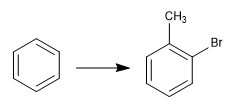
To obtain o-bromotoluene, we perform the following steps:
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 4836
The reaction of 1-chloro-2,4-dinitrobenzene with nucleophiles (hydroxide, ammonia, methoxide, etc.) produces the substitution of chlorine by the corresponding nucleophile. It is called ipso (same place), to indicate that the nucleophile occupies the same position as the starting chlorine.
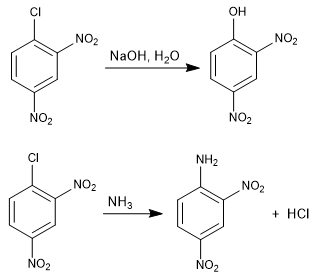
Read more: Aromatic nucleophilic substitution by addition-elimination
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 55922
Halogenated benzenes react with dilute soda under conditions of high pressure and temperature to form phenols. This reaction does not require deactivating groups in the ortho/para position and follows a different mechanism than aromatic nucleophilic substitution by addition-elimination.
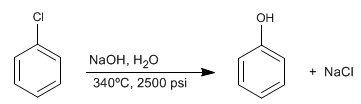
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 6311
The carbon attached directly to benzene is known as the benzylic position. In this position, highly stable carbocations, carbanions and radicals are formed due to the possibility of delocalizing the charge on the aromatic ring.
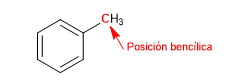
SN1 in benzylic positions
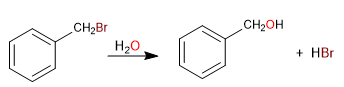
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 6527
Chain oxidation with permanganate and dichromate
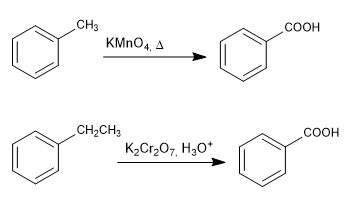
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 4941
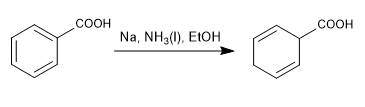
The Birch reduction uses sodium or lithium in solution as reagents, its mechanism is radical and reduces benzene to 1,4-cyclohexadiene.
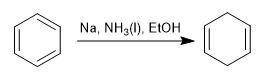
Birch with activating substituents
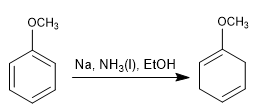
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 3852
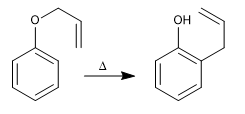
Allyl phenyl ethers undergo a concerted reaction when heated, involving the movement of six electrons, called the Claisen rearrangement. The intermediate formed in the reaction is of high energy and rapidly tautomerizes to give the final product.
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 4274
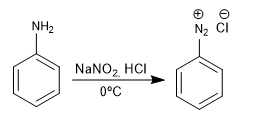
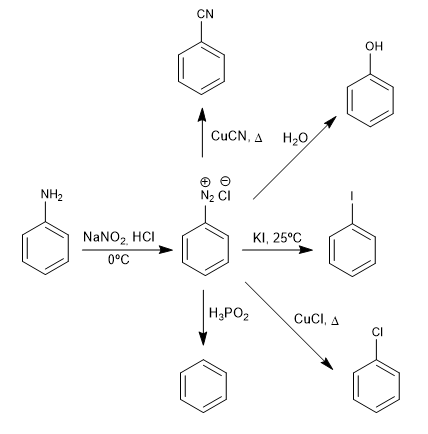
The benzenediazonium salts are attacked by nucleophiles in the presence of copper (I) salts that act as a catalyst, obtaining a wide variety of products.
- Details
- Germán Fernández
- THEORY OF BENZENE
- Hits: 5945
Formation of azo compounds