THEORY OF DIFUNCTIONAL COMPOUNDS
- Details
- Germán Fernández
- THEORY OF DIFUNCTIONAL COMPOUNDS
- Hits: 5797
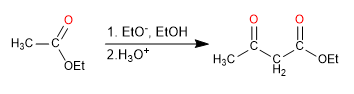
The 3-keto esters or β -keto esters are obtained by the Claisen condensation of two esters.
- Obtaining 3-ketoesters
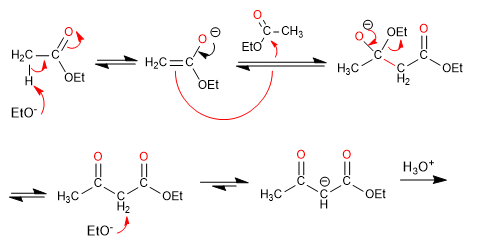
Mechanism:
- Details
- Germán Fernández
- THEORY OF DIFUNCTIONAL COMPOUNDS
- Hits: 35466
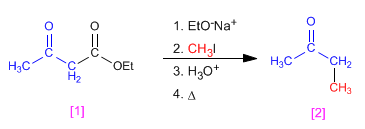
Ethyl 3-oxobutanoate (ethyl acetylacetate), can be obtained by Claisen from ethyl acetate, and is a very efficient reagent for the preparation of ketones.
- Details
- Germán Fernández
- THEORY OF DIFUNCTIONAL COMPOUNDS
- Hits: 47300
Malonic synthesis is a method that allows obtaining carboxylic acids. This synthesis starts with diethyl propanedioate (diethyl malonate).
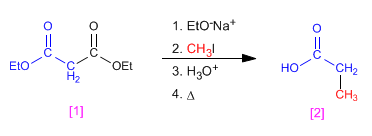
- Details
- Germán Fernández
- THEORY OF DIFUNCTIONAL COMPOUNDS
- Hits: 4291
When the electrophile used in the acetylacetic or malonic synthesis is a,b -unsaturated, a 1,5-dicarbonyl will be formed, this type of reaction is called Michael addition.
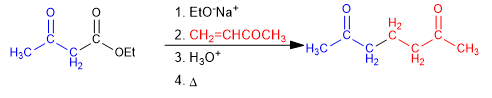
- Details
- Germán Fernández
- THEORY OF DIFUNCTIONAL COMPOUNDS
- Hits: 4325
The use of aldehydes and ketones in the acetylacetic or malonic synthesis allows obtaining ketones or a,b -unsaturated acids.
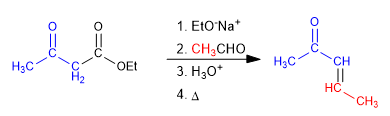
- Details
- Germán Fernández
- THEORY OF DIFUNCTIONAL COMPOUNDS
- Hits: 3968
Acyloinic condensation transforms esters into alpha-hydroxyketones. This reaction is carried out with sodium metal in an inert solvent.
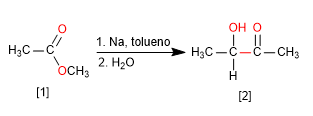
- Details
- Germán Fernández
- THEORY OF DIFUNCTIONAL COMPOUNDS
- Hits: 3937
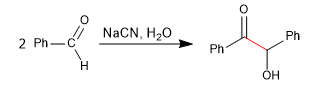
Benzoin condensation yields a -hydroxycarbonyls by reaction of aromatic aldehydes with sodium cyanide.
- Details
- Germán Fernández
- THEORY OF DIFUNCTIONAL COMPOUNDS
- Hits: 5624
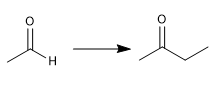
The reaction of aldehydes with 1,3-dithiacyclohexane, in the presence of Lewis acids, produces 1,3-dithianes that have hydrogens that can be subtracted with strong bases, forming the 1,3-dithiane anion. These anions are very good nucleophiles and react with a wide range of electrophiles.
- Details
- Germán Fernández
- THEORY OF DIFUNCTIONAL COMPOUNDS
- Hits: 4669
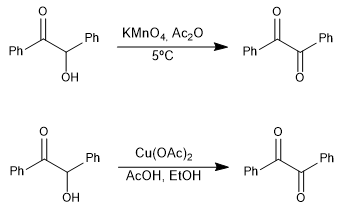
1,2-Dicarbonyl compounds can be prepared by oxidation of α-hydroxycarbonyls.