Alkanes are compounds made up of carbon and hydrogen that only contain carbon-carbon single bonds. They comply with the general formula CnH2n+2 , where n is the number of carbons in the molecule.
Alkanes in which the carbons are linked continuously (without branches) are called straight-chain alkanes .
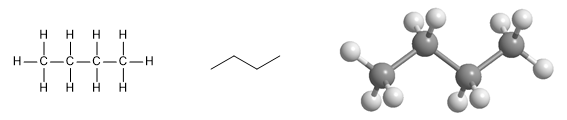
Butane molecule. The first drawing shows the form of union of the atoms, in the second the zig-zag shape of the molecule and finally its molecular model, where the spatial arrangement of the atoms is observed.
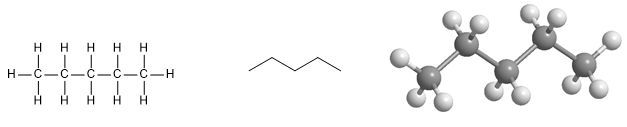
Pentane molecule. It is a linear alkane with five carbon atoms, which are arranged in a zig-zag pattern to reduce repulsions.
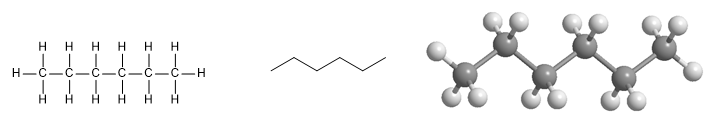
Hexane molecule. Hexane is a linear alkane of seven carbon atoms.
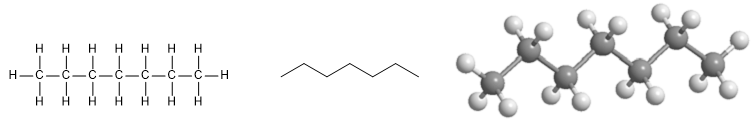
Heptane molecule
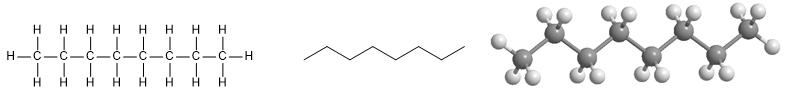
Octane molecule.
The family of linear alkanes is an example of a homologous series . Homologous series of compounds is one in which successive members differ by one methylene (CH 2 ) group. The general formula for homologous alkanes is CH3(CH2)nCH3 .
Propane (CH3CH2CH3 , with n=1) and butane (CH3CH2CH2CH3 , with n=2) are homologous.
In a homologous series the physical properties vary continuously, both the melting and boiling points increase as the number of carbons in the molecule increases.
Alkanes with branches are called branched-chain alkanes . 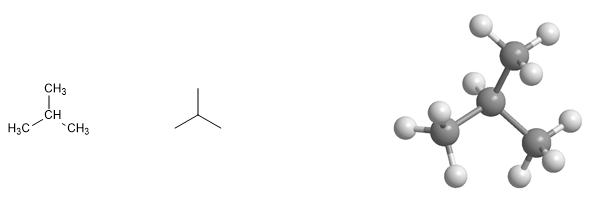
2-Methylpropane molecule. Methylpropane is a branched alkane, it has a linear chain of three carbon atoms and a branch in position 2 of the methyl type.
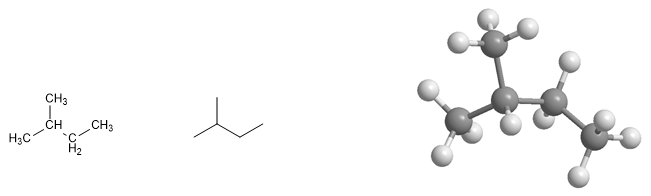
2-Methylbutane molecule. Methylbutane is a branched alkane with a straight chain of four carbon atoms and a branch at position 2.
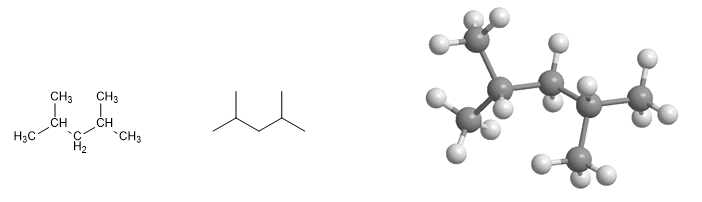
2,4-Dimethylpentane molecule. It presents ramifications at positions 2,4.