THE ORIGIN OF ORGANIC CHEMISTRY
- Details
- Germán Fernández
- Hits: 604148

The term "organic chemistry" was introduced in 1807 by Jöns Jacob Berzelius, to study compounds derived from natural resources. It was believed that compounds related to life possessed a "vital force" that made them different from inorganic compounds, in addition to being he considered impossible the preparation in the laboratory of an organic compound, which had been achieved with inorganic compounds.
WHAT DOES ORGANIC CHEMISTRY STUDY?
- Details
- Germán Fernández
- Hits: 925851
Organic chemistry is the scientific discipline that studies the structure, properties, synthesis and reactivity of chemical compounds formed mainly by carbon and hydrogen, which may contain other elements, generally in small amounts such as oxygen, sulfur, nitrogen, halogens, phosphorus, silicon. .
ORGANIC CHEMISTRY FORUM
- Details
- Germán Fernández
- Hits: 292183
This website provides its users with a consultation forum. To create an entry it is necessary to be registered and access the web, for this you must use the login block that appears in the left column.
Once inside the web, we click on the "new topic" tab, we choose the appropriate category for our query, we add a title that indicates to the rest of the users the topic to be treated and we add the necessary images using the "add file" button.
Access the forum (Recent Topics) (Only spanish)
ORGANIC CHEMISTRY COURSE
- Details
- Germán Fernández
- Hits: 291210
The ORGANIC CHEMISTRY section deals with the following topics:
Alkanes, cycloalkanes, radical halogenation reactions, stereochemistry, Alkenes, alkene reactions, alkynes, allylic systems, Diels Alder reaction, alcohols, ethers, aldehydes and ketones, reactions that proceed through enols and enolates, benzene, carboxylic acids, acid derivatives, amines, silicon compounds, phosphorus and sulfur.
Access to the organic chemistry I course
Access to the course videos (To have access to all the videos it is necessary to join channel members, only spanish)
ADVANCED ORGANIC COURSE
- Details
- Germán Fernández
- Hits: 266569
In this section the following advanced organic topics are studied:
1. Natural products
2. Mechanisms of reaction
3. Substitution and elimination reactions
4. Organic synthesis.
5. Oxidation reduction reactions
Access the advanced organic course
STRUCTURAL DETERMINATION
- Details
- Germán Fernández
- Hits: 246487

This section covers the following topics on determining organic structures:
1. Determination of organic compounds
2. Visible-ultraviolet spectroscopy
3. Infrared spectroscopy
4. NMR Spectroscopy
5. Mass spectrometry.
Access the structural determination section
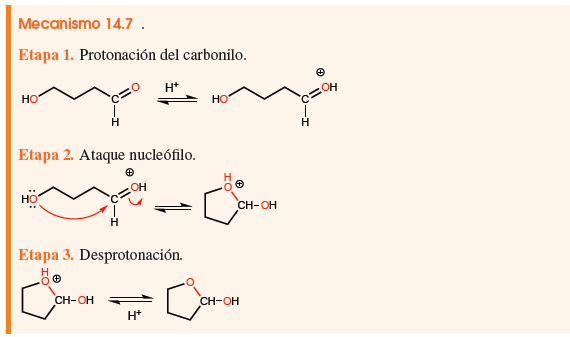
ORGANIC SYNTHESIS
- Details
- Germán Fernández
- Hits: 556266
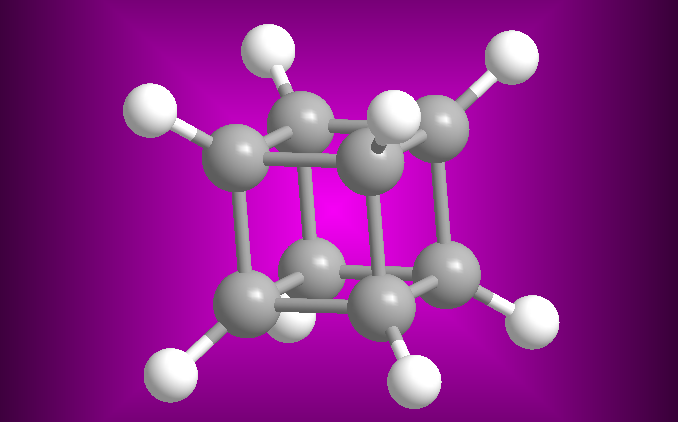
The synthesis of compounds is one of the most important parts of organic chemistry. The first organic synthesis dates back to 1828, when Friedrich Wöhler obtained urea from ammonium cyanate. Since then more than 10 million organic compounds have been synthesized from simpler compounds, both organic and inorganic.
Access the Organic Synthesis section created and maintained by Professor Wilbert Rivera Muñoz
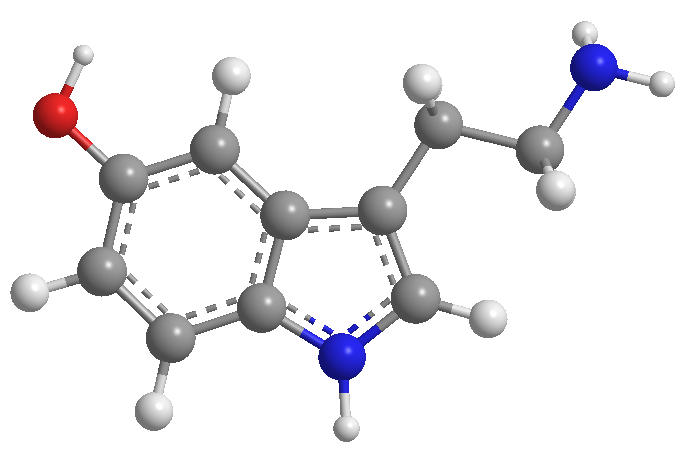
HETEROCYCLES COURSE
- Details
- Germán Fernández
- Hits: 11825
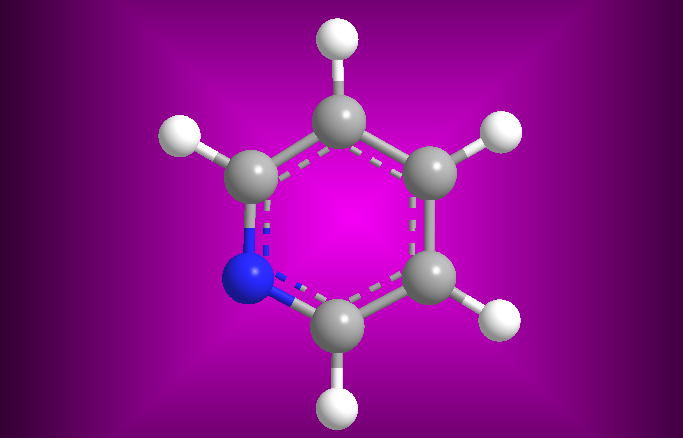
In this section we introduce the basic concepts of heterocyclic chemistry. We will start with the nomenclature according to the Hantzsch-Widman system, to continue with the study of the most relevant heterocyclic systems, pyridine, quinoline and isoquinoline, pyrrole, thiophene, furan and indole.
Access the heterocyclic chemistry course
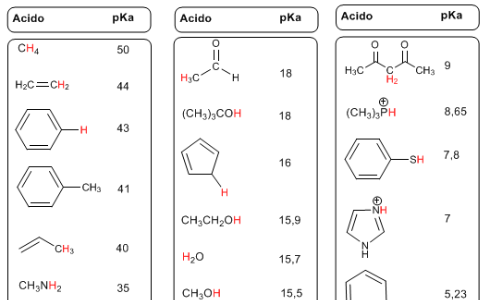
pKas LIST
- Details
- Germán Fernández
- Hits: 382369
The pKa is defined as -logKa, and indicates the degree of acidity of the hydrogens of an organic compound.
The hydrogens with respect to esters, amides, carboxylic acids, aldehydes, ketones... present an intermediate acidity with pKa values between 20 and 30. The protonated species have hydrogens with negative pKas, reaching values below -10 .
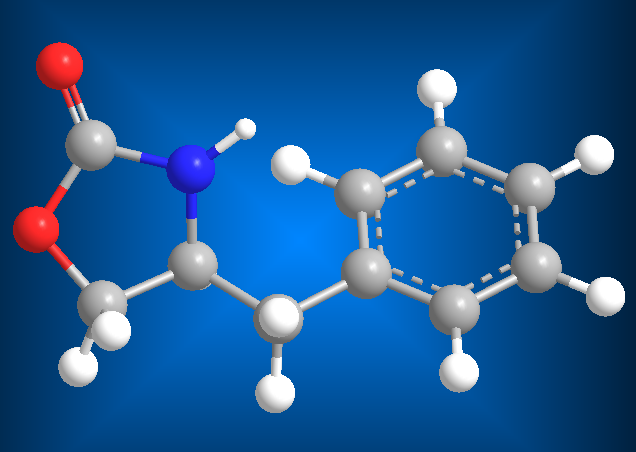
MOLECULAR MODELS
- Details
- Germán Fernández
- Hits: 426941
Molecular models are of great importance in organic chemistry, helping to predict properties and reactivity of organic compounds.
Here you will find models of organic compounds (balls, rods, surfaces), visualized using jmol , an interactive application programmed in java.
JSmol is a free and open source molecule viewer for students, teachers, and researchers in chemistry and biochemistry. It is cross-platform, compatible with Windows, Mac OS X and Linux/Unix systems.
VIDEO OF THE WEEK
- Details
- Germán Fernández
- Hits: 11423
Determination of the structure of an organic compound of formula C 6 H 10 O from its NMR and infrared spectra.
ORGANIC CHEMISTRY YouTube Channel
- Details
- Germán Fernández
- Hits: 78613
I have created this new channel specifically for organic chemistry, so the content will not be mixed with other subjects. In it we will deal with the typical topics of general organic chemistry as well as topics of advanced organic and heterocycles.
I hope you find it useful to prepare organic chemistry courses.
German Fernandez.
MOLECULAR EDITORS
- Details
- Germán Fernández
- Hits: 484775
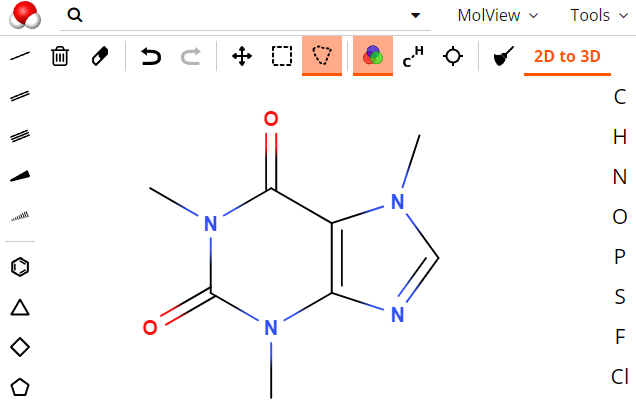
Molecular editors are computer programs designed to draw molecules and reactions. They are essential to be able to express themselves in organic chemistry, allowing to write reactions and mechanisms.
There are two molecular editors whose features stand out from the rest: ACD/ChemSketch and ChemDraw.
ANDROID APPS
- Details
- Germán Fernández
- Hits: 164706
Android applications that you can download on Google Play
Organic Chemistry: application that contains a large part of the content shown on this website
https://play.google.com/store/apps/details?id=com.net.quimicaorganica&hl=es
Organic Chemistry Nomenclature: application with theory and interactive exercises to name organic compounds.
https://play.google.com/store/apps/details?id=german.nomenclatura_organica&hl=es