SULFUR, PHOSPHORUS AND SILICON
- Details
- Germán Fernández
- SULFUR, PHOSPHORUS AND SILICON
- Hits: 2249
Thiols are characterized by containing the -SH functional group. They are named by ending the main chain name in -thiol, analogous to alcohols whose ending is -ol
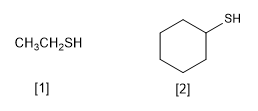
[1] Ethanethiol
[2] Cyclohexanethiol
Thiols have a higher acidity than alcohols due to the larger size of sulfur compared to oxygen. The pKa values are around 10-11, compared to alcohols that have values between 16-18.
- Details
- Germán Fernández
- SULFUR, PHOSPHORUS AND SILICON
- Hits: 3278
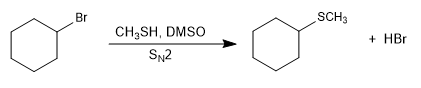
They are compounds similar to ethers in which oxygen is replaced by sulfur (RSR). They are also called sulfides.
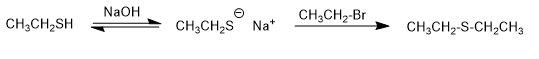
- Details
- Germán Fernández
- SULFUR, PHOSPHORUS AND SILICON
- Hits: 2444
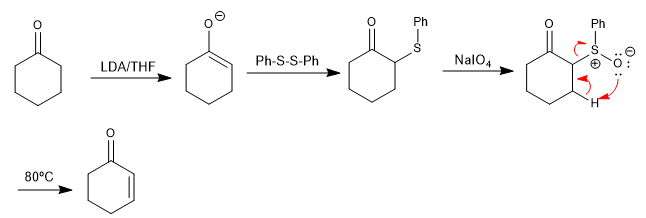
Sulfoxides undergo elimination reactions, under slight heating, to form alkenes. The sulfoxide can be easily prepared by oxidation of a thioether, which is easy to form at the alpha position of a carbonyl from a disulfide. This procedure allows the preparation of α,β-unsaturated carbonyls.
- Details
- Germán Fernández
- SULFUR, PHOSPHORUS AND SILICON
- Hits: 3518
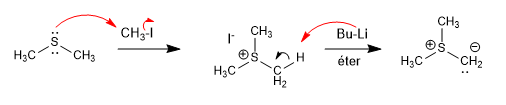
Sulfur ylides are prepared from thioethers by alkylation, followed by deprotonation.
- Details
- Germán Fernández
- SULFUR, PHOSPHORUS AND SILICON
- Hits: 6341
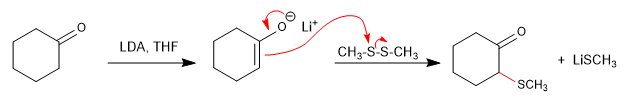
The reaction of carbonyls with thiols forms thioacetals that can be used as protecting groups or to reduce the carbonyl to an alkane.
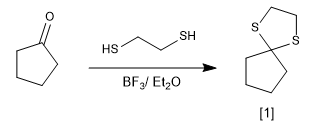
thioacetal
Thioacetal is stable in acid media but can be hydrolyzed with mercury(II) salts.
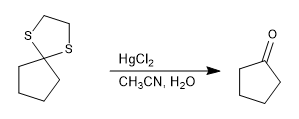
- Details
- Germán Fernández
- SULFUR, PHOSPHORUS AND SILICON
- Hits: 6014
The 1,3-dithianes allow to change the polarity of the carbonyl carbon of the aldehydes by subtraction of the acid hydrogen, obtaining an organolithic capable of attacking a wide variety of electrophiles.
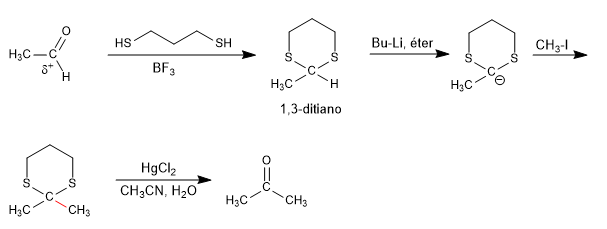
The initial carbonyl, with positive polarity on carbon, changes in the umpolung reaction to a carboanion. The sulphurs of 1,3-dithiane are vital in stabilizing the negative charge, and the reaction with the oxygenated equivalent is not viable.
- Details
- Germán Fernández
- SULFUR, PHOSPHORUS AND SILICON
- Hits: 3955
Phosphines are obtained by reacting phosphorus trichloride with organometallic reagents. Thus, phosphorus trichloride reacts with phenylmagnesium bromide to form triphenylphosphine.
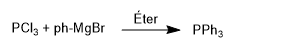
This reaction is well controlled and allows only two groups to be introduced.