In 1929, Professor S. Kharasch of the University of Chicago observed the anti-Markovnikov addition of HBr to an alkene due to the presence of peroxides in the reaction medium.

Bromine is added to the less substituted carbon of the alkene, while hydrogen is attached to the carbon with more substituents (antiMarkovnikov)
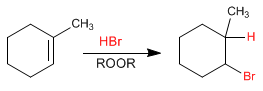
In the absence of peroxides, the addition of HBr to the alkene is Markovnikov, that is, the bromine adds to the most substituted carbon.
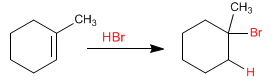
The addition of hydrogen bromide to an alkene follows two different mechanisms depending on the presence or absence of peroxides in the reaction medium.
In the absence of peroxides, a carbocation forms at the most substituted position of the alkene.
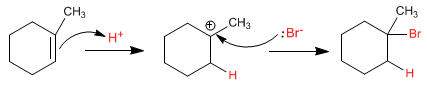
In the presence of peroxides, a radical mechanism is followed, with formation of a radical-type intermediate on the most substituted carbon of the alkene.
Let's see the mechanism of the following global reaction:
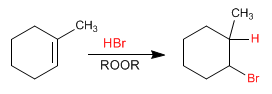
a) initiation
The initiation consists of two stages:
Stage 1. The peroxide dissociates into two alkoxy radicals.
Stage 1. The peroxide dissociates into two alkoxy radicals.
Stage 2. Abstraction of hydrogen from hydrogen bromide by the alkoxy radical, forming bromine radicals that pass to the propagation stage.
b) Propagation
Propagation consists of two stages:
Stage 3. The bromo radical is added to the alkene.
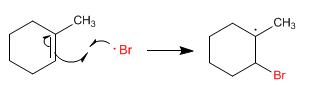
Stage 4. The radical formed on carbon abstracts hydrogen from hydrogen bromide, forming new bromine radicals.
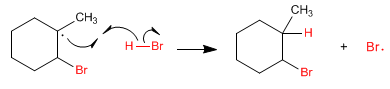
This addition is anti-Markovnikov since the radical formed in step 3 is tertiary (highly stabilized by hyperconjugation). A Markovnikov addition would generate a much more unstable secondary radical.