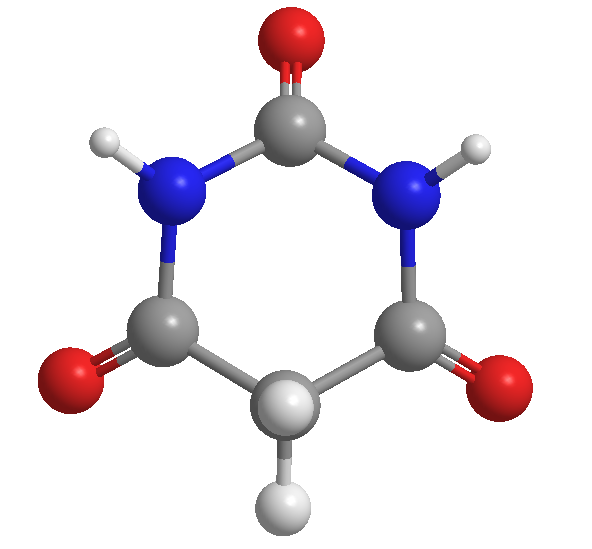
Barbituric acid, discovered by Adolf von Baeyer, in 1864
In the origins of chemistry, organic compounds were named by their discoverers. Urea gets its name from being isolated from urine.
Barbituric acid was discovered by the German chemist Adolf von Baeyer in 1864. It is speculated that he gave it this name in honor of a friend named Barbara.
Chemical science was advancing and the large number of organic compounds discovered made it essential to use a systematic nomenclature.

[1] Isobutane (common name); Methylpropane (IUPAC name)
[2] Isopentane (common name); Methylbutane (IUPAC name)
In the IUPAC system of nomenclature, a name is made up of three parts: prefixes, main, and suffixes; The prefixes indicate the substituents of the molecule; the suffix indicates the functional group of the molecule; and the main part the number of carbons it has.
Alkanes can be named following seven steps:
Rule 1.- Determine the number of carbons of the longest chain, called the main chain of the alkane. Observe in the figures that it is not always the horizontal chain.
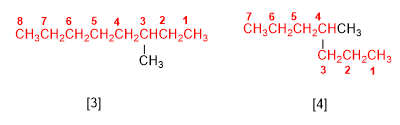
[3] 3-methyloctane
[4] 4-methylheptane
The name of the alkane ends in the name of the main chain (octane, heptane) and is preceded by the substituents.
Rule 2.- The substituents are named by changing the ending -ane of the alkane from which they are derived by -yl (methyl, ethyl, propyl, butyl). In the name of the alkane, the substituents precede the name of the main chain, and are accompanied by a locant that indicates their position within the main chain. Backbone numbering is done so that the substituent is assigned the lowest possible locant. 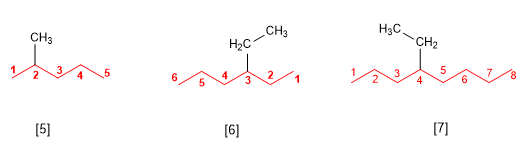
[5] 2-methylpentane
[6] 3-ethylhexane
[7] 4-ethyloctane
Rule 3.- If we have several substituents, they are ordered alphabetically preceded by the locators. The numbering of the main chain is done so that the substituents together take the minor locants.
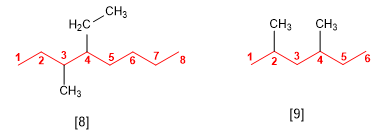
[8] 4-ethyl-3-methyloctane
[9] 2,4-Dimethylhexane
If several substituents are the same, the prefixes di, tri, tetra, penta, hexa are used to indicate the number of times each substituent occurs in the molecule. Locants are separated by commas and there must be as many as there are substituents. 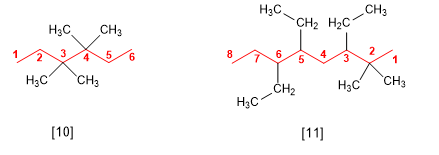
[10] 3,3,4,4-Tetramethylhexane
[11] 3,5,6-Triethyl-2,2-dimethyloctane
Quantity prefixes are not taken into account when sorting alphabetically.
Rule 4.- If when numbering the main chain at both ends, we find ourselves at the same distance with the first substituents, we look at the other substituents and number them so that they take the minor locants. 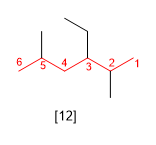
[12] 3-Ethyl-2,5-dimethylhexane
Rule 5.- If by numbering in both directions the same locants are obtained, the lowest locant is assigned to the substituent that comes first in alphabetical order.
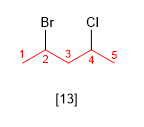
[13] 2-Bromo-4-chloropentane
Rule 6.- If two or more chains have the same length, the one with the greatest number of substituents is taken as the main one. 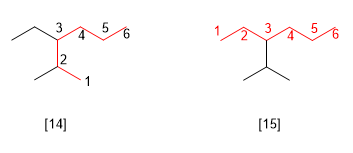
[14] 3-Ethyl-2-methylhexane (correct)
[15] 3-Isopropylhexane (incorrect)
Rule 7.- There are some substituents with common names accepted by the IUPAC, although the use of systematic nomenclature is recommended. 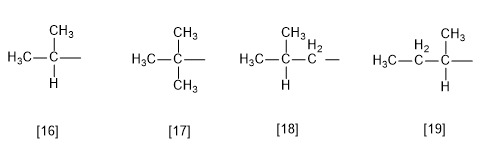
[16] Isobutyl (1-methylethyl)
[17] tert-butyl (1,1-dimethylethyl)
[18] Isobutyl (2-methylpropyl)
[19] sec-butyl (1-methylpropyl)
The systematic names of these substituents are obtained by numbering the chain beginning with the carbon that joins the parent. The name of the substituent is formed with the name of the longest chain ending in -ilo, preceding the names of the substituents that this secondary chain has in alphabetical order. Let's see an example:
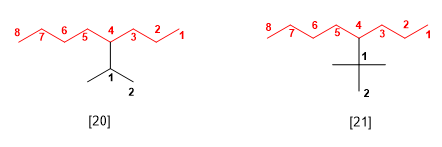
[20] 4-Isopropyloctane (4-(1-methylethyl)octane)
[21] 4-tert-butyloctane (4-(1,1-dimethylethyl)octane)
Table of alkanes with less than 10 carbon atoms.
Methane (CH4)
Ethane (CH3CH3)
Propane (CH3CH2CH3)
Butane (CH3CH2CH2CH3)
Pentane (CH3CH2CH2CH2CH3)
Hexane (CH3CH2CH2CH2CH2CH3)
Heptane (CH3CH2CH2CH2CH2CH2CH3)
Octane (CH3CH2CH2CH2CH2CH2CH2CH3)
Nonane (CH3CH2CH2CH2CH2CH2CH2CH2CH3)
Decane (CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3)