ETHER THEORY
- Details
- Germán Fernández
- ETHER THEORY
- Hits: 96672
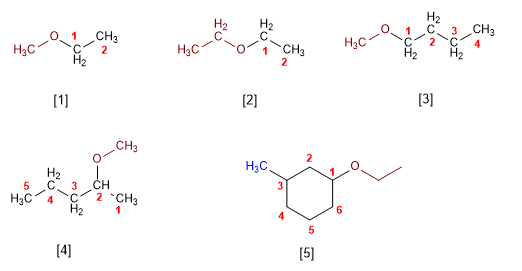
Rule 1. Ethers can be named as alkoxy derivatives of alkanes (substitutive IUPAC nomenclature). The longest chain is taken as the main chain and the alkoxide is named as a substituent.
- Details
- Germán Fernández
- ETHER THEORY
- Hits: 71455
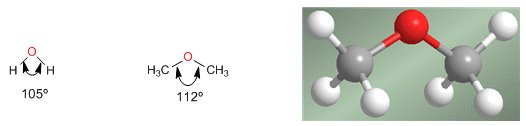
Ethers are molecules similar in structure to water and alcohols. The angle between the C-O-C bonds is greater than in water due to steric repulsions between bulky groups.
- Details
- Germán Fernández
- ETHER THEORY
- Hits: 176677
Ethers have lower boiling points than alcohols, although their solubility in water is similar. Given their significant stability in basic media, they are used as inert solvents in numerous reactions.
- Details
- Germán Fernández
- ETHER THEORY
- Hits: 97651
- Details
- Germán Fernández
- ETHER THEORY
- Hits: 105503
The reaction between a primary haloalkane and an alkoxide (or alcohol in a basic medium) is the most important method for preparing ethers. This reaction is known as the Williamson synthesis.
- Details
- Germán Fernández
- ETHER THEORY
- Hits: 4917
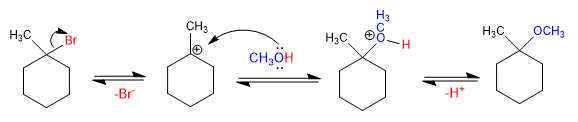
Ethers can be obtained by reacting haloalkanes with alcohols through the S N 1 mechanism. The haloalkane must be secondary or tertiary to form stable carbocations.
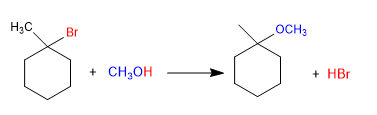
Mechanism:
- Details
- Germán Fernández
- ETHER THEORY
- Hits: 3801
Alcohols can be protected by transforming them into ethers. This process is carried out by reacting the alcohol to be protected with tert-butanol in a sulfuric acid medium. The deprotection takes place in an aqueous acid medium. Let's see an example:
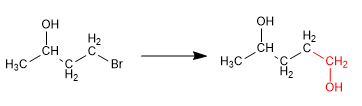
In this synthesis it is necessary to form a carbon-carbon bond using organometallic reagents, incompatible with alcohol. Thus, the alcohol must be previously protected to avoid the decomposition of the organometallic.
- Details
- Germán Fernández
- ETHER THEORY
- Hits: 4073
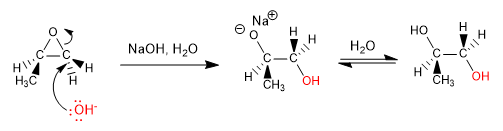
Epoxides (oxacyclopropanes) are three-membered cyclic ethers. Its main characteristic is the tension of the ring, which favors its opening in both basic and acidic media.
Opening in basic media: Epoxides open by nucleophile attack on the least substituted carbon (opening is governed by steric hindrances)