The a,b -unsaturated are compounds that have two electrophilic positions: the carbonyl carbon and the b carbon.
Additions 1,2. Lithium organometallics attack the carbonyl carbon giving rise to 1,2 additions. 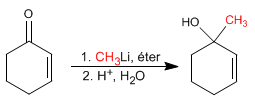
![]() The organometallics of lithium and magnesium attack the carbonyl carbon of the a,b-unsaturates
The organometallics of lithium and magnesium attack the carbonyl carbon of the a,b-unsaturates
Addition Mechanism 1.2 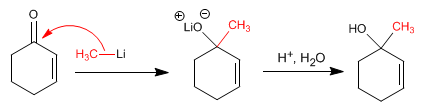
Additions 1.4. Cuprates, cyanide, and other nucleophiles attack the b carbon of a,b -unsaturates, giving 1,4 additions.![]() Hydrocyanic acid gives 1,4 additions with the a,b -unsaturated. The cyano is attached to carbon b.
Hydrocyanic acid gives 1,4 additions with the a,b -unsaturated. The cyano is attached to carbon b.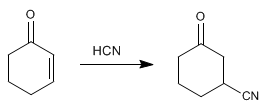
Mechanism of addition of hydrocyanic acid to Cyclohex-2-enone 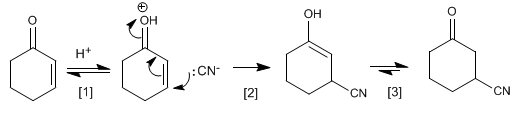
carbonyl protonation
Cyanide nucleophilic attack on carbon b .
Keto-enol tautomerism.![]() Cuprates are copper organometallics that add to the b carbon of a,b -unsaturated compounds.
Cuprates are copper organometallics that add to the b carbon of a,b -unsaturated compounds.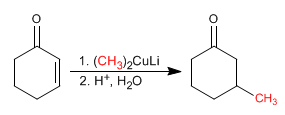
The reaction mechanism begins with the nucleophilic attack of the cuprate on the b carbon, forming an enolate, which is protonated in the second stage to give an enol. The enol tautomerizes to a ketone, generating the final product. 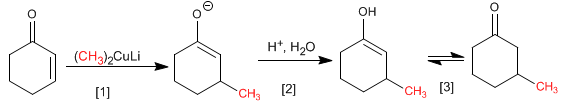
[1] Nucleophilic addition of cuprate.
[2] Enolate protonation
[3]Keto–enol tautomerism