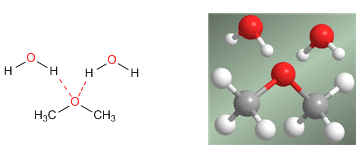
Ethers have lower boiling points than alcohols, although their solubility in water is similar. Given their significant stability in basic media, they are used as inert solvents in numerous reactions.
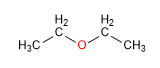
Diethyl ether
Boiling point = 35ºC
Water solubility = 7.5g/100ml
The important solubility in water is explained by the hydrogen bonds that are established between the hydrogens of the water and the oxygen of the ether.