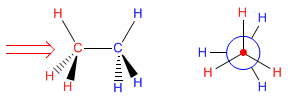
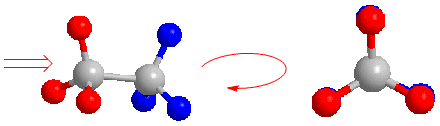
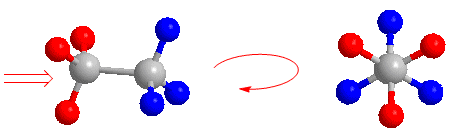
 The Newman projection is obtained by looking at the molecule along the CC axis. The front carbon is represented by a point, from which the three bonds that join it to the substituents start. The carbon behind is represented by a circle, and the bonds leaving this carbon are drawn from this circle.
The Newman projection is obtained by looking at the molecule along the CC axis. The front carbon is represented by a point, from which the three bonds that join it to the substituents start. The carbon behind is represented by a circle, and the bonds leaving this carbon are drawn from this circle.
Next, we will draw the Newman projection of alternating ethane. We look at the molecule by placing ourselves in the position of the arrow. We represent the carbon in front of us by a point and we draw the bonds that start towards the hydrogens. We cannot see the carbon that is at the bottom, although we can see the hydrogens that come from it (represented in red). We represent it by a circle and we draw the bonds that join it to the hydrogens (represented in blue). 
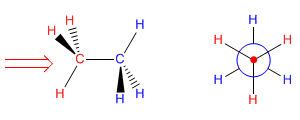
Now let's try to project the conformation of ethane that has all the hydrogens facing each other (called the eclipsed conformation). Placing ourselves in the position of the arrow we have in front of the carbon with the red hydrogens. The blue hydrogens are covered and we cannot see them. In the Newman projection these hydrogens (blue) appear slightly rotated in order to represent them.