STEREOCHEMISTRY THEORY
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 1932
Isomer definition
Molecules that have the same molecular formula but different structures are called isomers. It is classified as structural isomers and stereoisomers.
Structural isomers
Structural isomers differ in the way the atoms are joined and are in turn classified into positional and functional chain isomers.
Stereoisomers
Stereoisomers have all the identical bonds and are differentiated by the spatial arrangement of the groups. They are classified as cis-trans or geometric isomers, enantiomers and diastereoisomers.
Chiral or asymmetric center
An atom bonded to four different substituents is called a chiral or asymmetric center. A molecule that has a chiral center has a non-superimposable mirror image of it, called an enantiomer.
Optical activity
The enantiomers have almost all identical physical properties, with the exception of optical activity. One of the enantiomers rotates polarized light to the right (right-handed) and the other rotates polarized light to the left (left-handed).
Stereochemistry in reactions
Radical halogenation reactions on molecules in which chiral centers can be formed produce mixtures of enantiomers in equal amounts or of diastereoisomers in different proportions.
Separation of enantiomers
Enantiomers have almost all the same physical properties, differ in rotation polarized light, but have the same melting and boiling points and identical solubility. Therefore, we cannot apply the traditional methods of separation and we must resort to special techniques. The separation via diastereoisomers, consists of transforming the mixture of enantiomers into a mixture of diastereoisomers by adding a chiral reagent, the diastereoisomers are easily separated by physical methods.
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 109632
Stereochemistry is the study of organic compounds in space. To understand the properties of organic compounds it is necessary to consider all three spatial dimensions. 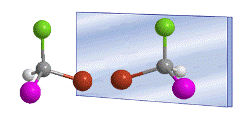 The bases of stereochemistry were laid by Jacobus van't Hoff and Le Bel, in 1874. They independently proposed that the four substituents of a carbon are directed towards the vertices of a tetrahedron, with the carbon in the center of it. .
The bases of stereochemistry were laid by Jacobus van't Hoff and Le Bel, in 1874. They independently proposed that the four substituents of a carbon are directed towards the vertices of a tetrahedron, with the carbon in the center of it. .
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 293293
Isomers are molecules that have the same molecular formula but different structures. It is classified as structural isomers and stereoisomers. Structural isomers differ in the way their atoms are bonded and are classified into chain, position, and function isomers. As an example, let's draw the structural isomers of formula C 2 H 6 O .

[1] Ethanol
[2] Dimethyl ether
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 263311
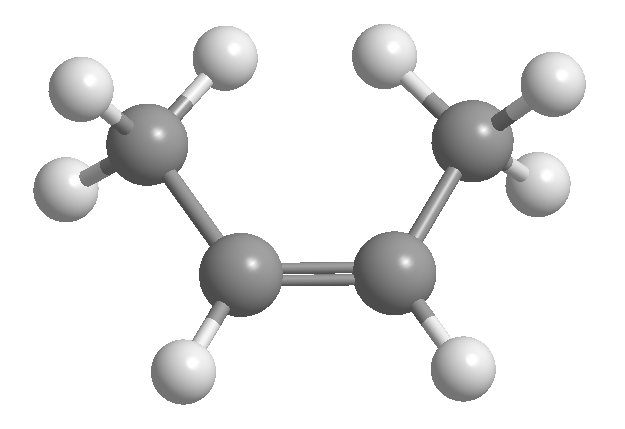
cis-trans or geometric isomerism is due to restricted rotation around a carbon-carbon bond. This restriction may be due to the presence of double bonds or cycles. Thus, 2-butene can exist in the form of two isomers, called cis and trans. The isomer with the hydrogens on the same side is called cis, and the one with the opposite sides is called trans.

[1] cis-2-Butene
[2] trans-2-Butene
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 160031
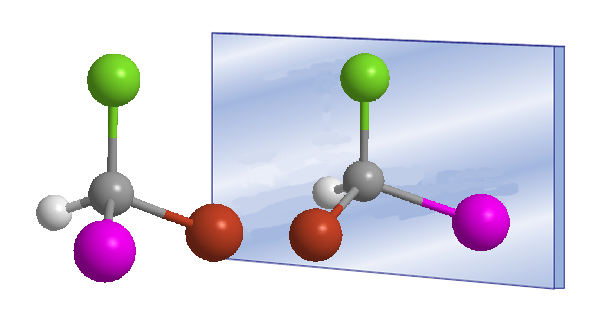
The word chiral was introduced by William Thomson (Lord Kelvin) in 1894 to designate objects that are not superimposable with their mirror image. Applied to organic chemistry, we can say that a molecule is chiral when it and its mirror image are not superimposable.
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 106378
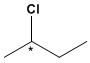 Compounds with an asymmetric carbon, such as 2-chlorobutane, can exist as two isomers.
Compounds with an asymmetric carbon, such as 2-chlorobutane, can exist as two isomers.
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 5896
The maximum number of stereoisomers that a molecule presents can be calculated with the formula (2n), where n represents the number of asymmetric carbons. Thus, a molecule with 2 chiral centers has 4 stereoisomers.
Example 1. Draw the possible stereoisomers of 2-Bromo-3-chlorobutane.
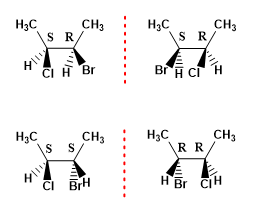
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 127964
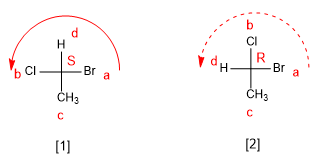
A nomenclature is necessary that distinguishes the stereoisomers of a molecule. Thus, in the case of 2-Chlorobutane the notation must distinguish one enantiomer from the other. Cahn, Ingold and Prelog developed some rules that allow us to distinguish some stereoisomers from others, which I describe below.
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 96022
Molecules that have a plane of symmetry or a center of inversion are superimposable with their mirror image. They are said to be achiral molecules.
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 132646
Optical activity is the ability of a chiral substance to rotate the plane of polarized light. It is measured using a device called a polarimeter.
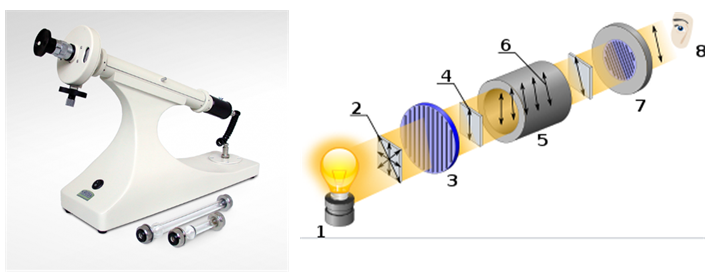
[1] Light source
[2] Unpolarized light
[3] Linear polarizer
[4] Linearly polarized light
[5] Sample cuvette
[6] Rotation in polarized light
[7] Analyzer
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 131891
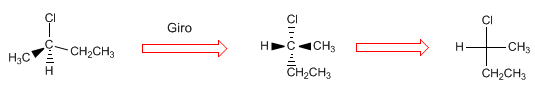
Projecting consists of drawing a molecule in two dimensions (plane). In the Fischer projection, the molecule is drawn in the shape of a cross with the substituents that go to the bottom of the plane in the vertical and the groups that come out towards us in the horizontal, the point of intersection of both lines represents the projected carbon.
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 91767
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 98661
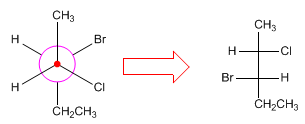
To convert Newman projections to Fischer projections, the spatial shape of the molecule is drawn, arranging it in an eclipsed conformation to make the Fischer projection.
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 90866
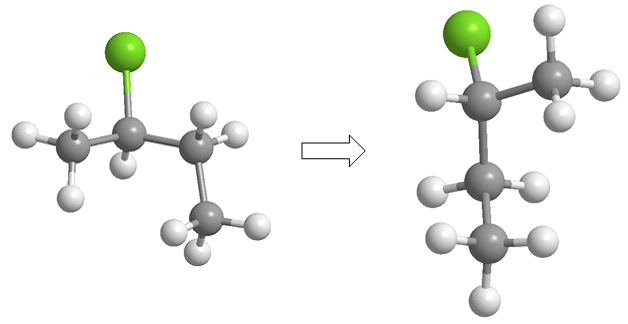
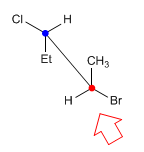 In the sawhorse projection (also called in perspective) the line of observation makes an angle of 45º with the carbon-carbon bond. The carbon closest to the observer is below and to the right. While the farthest one is on the top left.
In the sawhorse projection (also called in perspective) the line of observation makes an angle of 45º with the carbon-carbon bond. The carbon closest to the observer is below and to the right. While the farthest one is on the top left.
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 4980
We are going to see how chemical reactions can introduce chirality in molecules, obtaining products in the form of racemic mixtures or mixtures of diastereoisomers.
Butane halogenates in the presence of bromine and light, at carbon 2, to form a mixture of enantiomers. The radical formed presents enantiotopic faces, which are halogenated with equal probability, giving rise to a racemic mixture (enantiomers in equal proportion).
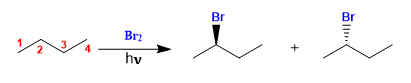
The mechanism of this reaction consists of three stages: initiation, propagation and termination. Propagation is the step that determines the stereochemistry of the final product.
Butane halogenation
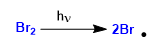
Stage 1. Initiation
Stage 2. Propagation
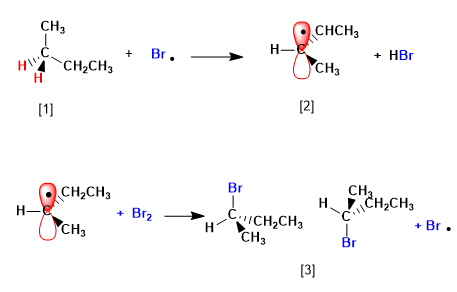
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 5097
Stereoselective reaction
- Details
- Germán Fernández
- STEREOCHEMISTRY THEORY
- Hits: 3858
Difficulties in separating racemates