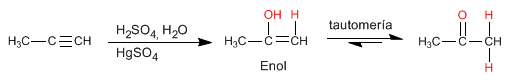
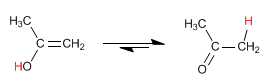Alkynes react with aqueous sulfuric acid in the presence of a mercury catalyst to form enols. The enol isomerizes (tautomerizes) rapidly under the reaction conditions to give aldehydes or ketones.
Stage 1. Electrophilic addition. The proton adds to the triple bond, joining the least substituted carbon.
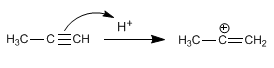
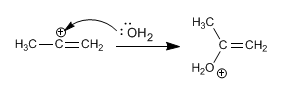
Stage 2. The water captures the carbocation formed in the previous step.
Stage 3. Deprotonation of the water, forming the enol
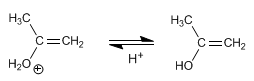
Stage 4. Keto-enol tautomerism