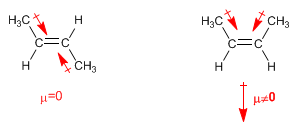Alkenes have melting and boiling points close to the corresponding alkanes.
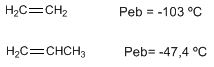
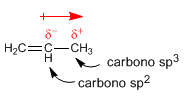
Dipole moment in alkenes. In carbon $sp^2$ the electrons in the s orbital are closer to the nucleus and are strongly attracted to it, so that a carbon $sp^2$ has a tendency to attract electrons towards itself, which generates dipole moments.
In " trans " alkenes the dipole moments are subtracted, even canceling out in the event that both carbons have the same chains. In cis alkenes the dipole moments add up giving rise to a non-zero total dipole moment (polar molecule)