ENOL AND ENOLATE PROBLEMS
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 12628
Explain why when a solution of (S)-3-phenyl-2-butanone in aqueous ethanol is treated with acids or bases it gradually loses its optical activity. (b) Why does the racemization of the compound from the previous section in an acid medium occur at the same speed as the halogenation in an acid medium? (c) Why does the iodination of said compound in an acid medium occur at the same rate as acid-catalyzed bromination?
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 8596
Treating cis -2-propyl-3-methyl-cyclopentanone with a weak base transforms it into its trans stereoisomer. Reason this phenomenon and draw the corresponding structures.
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 13134
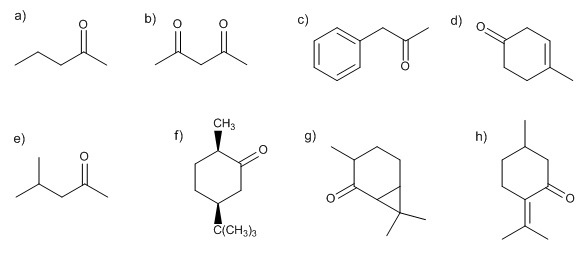
Write the structure of all the possible enolates of each ketone or aldehyde. Indicate which would be the most favored under kinetic or thermodynamic control. Explain.
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 27341
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 10248
Indicate the products (if any) with deuterium incorporation that are formed when treating the following compounds with D 2 O/NaOD.
(a). Cyclohexanone; (b) 2,2-Dimethylpropanal; (c) 3,3-Dimethyl-2-butanone; (d) 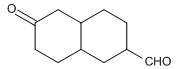
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 13579
Draw the products of acid- and base-catalyzed bromination of acetylcyclopentane. Indicate the major product. Reason your answer.
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 7925
Explain the fact that cyclohexanone enolate reacts with 2-bromobutane giving rise to two reaction products, while only one is formed with 2-bromo-2-methylpropane.
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 7426
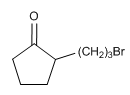 Reaction of the indicated compound with base provides three isomeric C 8 H 12 O products resulting from intramolecular alkylations. Indicate what they are.
Reaction of the indicated compound with base provides three isomeric C 8 H 12 O products resulting from intramolecular alkylations. Indicate what they are.
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 10032
Indicate the product(s) of each of the following reactions.
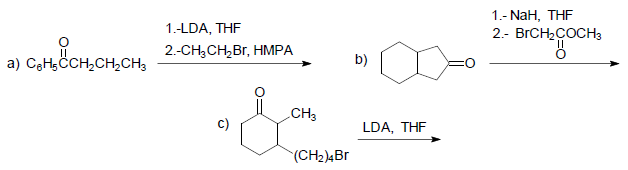
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 13859
Explain the following facts and propose a possible mechanism:
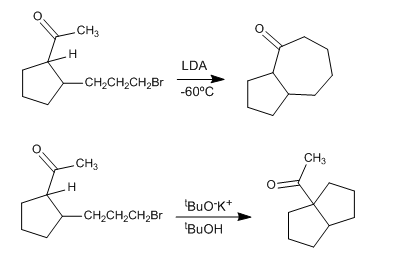
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 8787
Explain the following reactions and tell the structures of A, B, C, D, E and F.
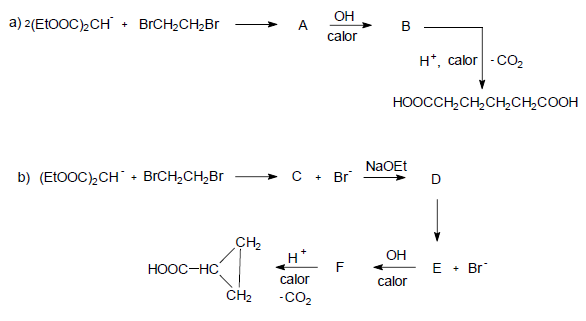
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 12653
Explain the following reaction and tell the structure of compounds A, B, C, D and E.
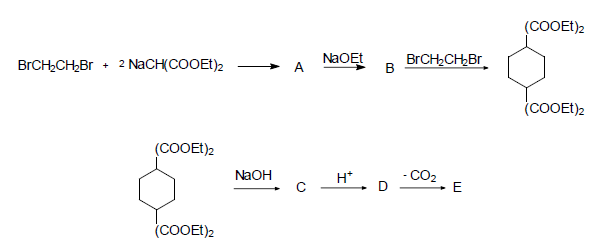
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 10261
Explain the mechanism of the following reaction.
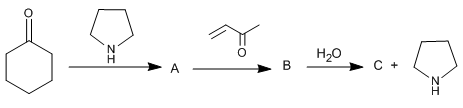
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 10734
It indicates a suitable synthetic procedure for obtaining 2,2-dimethylbutanal from 2-methylpropanal. 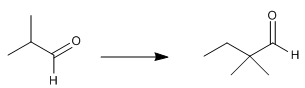
- Details
- Germán Fernández
- ENOL AND ENOLATE PROBLEMS
- Hits: 7944
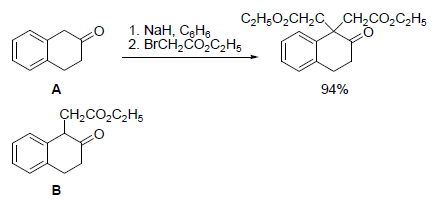
The alkylation of the enolate of ketone A is very difficult to stop before the dialkylation occurs, as indicated below. Show how the monoalkylated ketone B could be obtained.