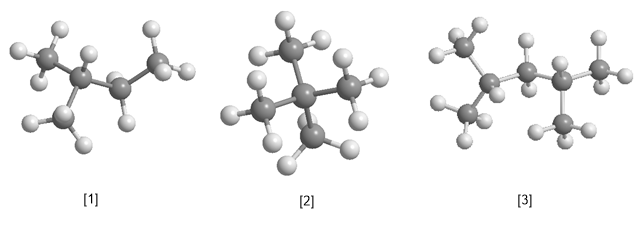
ALKANE PROBLEMS
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 49429
Write the condensed formula of a linear alkane, whose molecular formula is C20H42 .
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 34693
Give the molecular formula, the condensed formula, and indicate the number of hydrogens each carbon has in the octane molecule.
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 21495
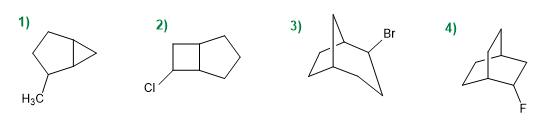
Indicate the number of carbons in each of the three chains that start from the bridgehead carbons in the following bicycles. 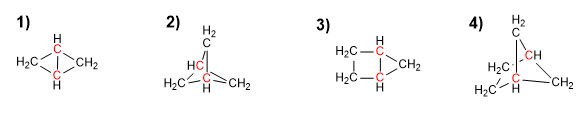
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 102822
Write the formulas and names of linear alkanes with fewer than ten carbons.
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 29626
Write the structural formula of 2,4,6-trimethyloctadecane.
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 103427
Write the molecular formula, the semideveloped formula and name the linear alkanes of 20, 30, 40, 50 and 100 carbons.
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 71117
Write and name the alkyl radicals with fewer than five carbons.
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 35217
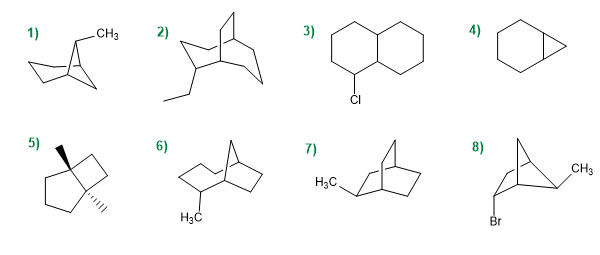
Give the IUPAC name of the following compounds. 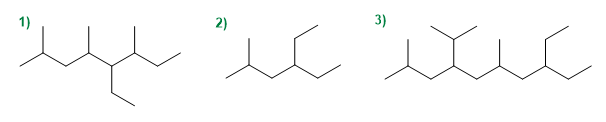
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 32141
Draw the structure of the following bicyclic compounds.
| 1) 2-Bromo-5-ethylbicyclo[2.1.1]hexane 2) Bicyclo[5.4.0]undecane 3) 1-Ethyl-4-methylbicyclo[4.4.0]decane 4) 2-Chlorobicyclo[2.2.2]octane 5) Bicyclo[1.1.0]butane | 6) Bicyclo[3.2.1]octane 7) 2-Isopropyl-5-methylbicyclo[2.2.1]heptane 8) Bicyclo[2.2.0]hexane 9) 2-Chloro-3-bromobicyclo[1.1.1]pentane 10) Bicyclo[2.1.0]pentane |
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 24759
Draw the most stable and least stable conformations of 2,3-dimethylpentane between carbons C3-C4.
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 42973
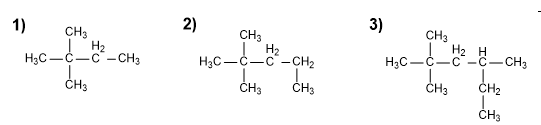
For the following branched alkanes, choose the main chain, number it, and name the compound. 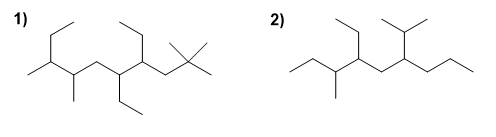
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 17613
Which of the following compounds can exist in different conformations?
a) Hydrogen peroxide.
b) Ammonia.
c) Methanol.
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 43554
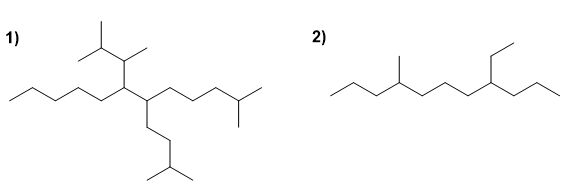
Give the IUPAC names of the following compounds: 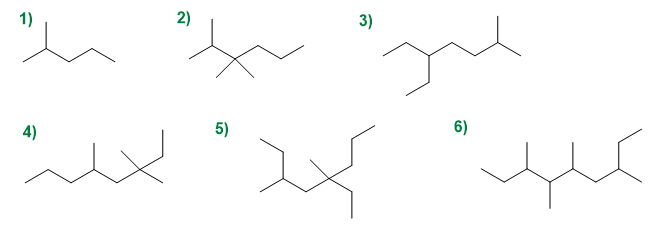
- Details
- Germán Fernández
- ALKANE PROBLEMS
- Hits: 76937
Write the structural formula for each of the following alkanes.
| 1) 6-Isopropyl-2,5-dimethylnonane 2) 4-tert-butyl-3-methylheptane 3) Pentacosane 4) 4-Ethyl-4-methylheptane 5) 2,3-Dimethylpentane |
6) 5,5-Diethyl-2-methyl-4-propyldecane 7) 2,3,4-Trimethyloctane. 8) 4-tert-Butyloctane 9) 3-Ethyl-6,7-dimethyl-4-propyldodecane 10) 4,5-Diethyl-5-isopropyl-3,4-dimethyl-6-propylundecane |