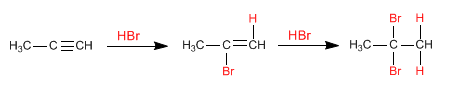
Similar to alkenes, alkynes add hydrogen halides (HBr, HCl, HI) to the triple bond to form alkenyl halides.
The reaction mechanism occurs through a carbocation, formed on the most substituted carbon of the triple bond. Therefore, it is a regioselective reaction that follows the Markovnikov rule, adding the halogen to the most substituted carbon of the alkyne.
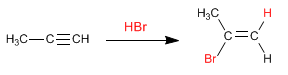
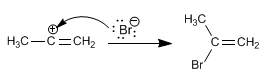
Stage 1. Electrophilic addition. The proton adds to the triple bond, joining the least substituted carbon.
Stage 2. The halogen captures the carbocation formed in the previous step.
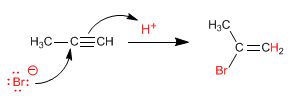
Given the great instability of the carbocation formed (alkenyl cation) the previous mechanism is questioned. Another alternative mechanism is the concerted one (a single stage, without the formation of intermediates).
In the presence of two equivalents of the hydrogen halide, successive additions to the alkyne occur, forming geminal dihaloalkanes.