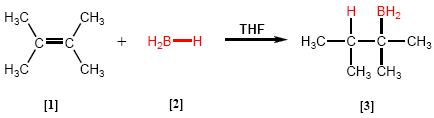
Hydroboration is a reaction in which a boron hydride [2] reacts with an alkene [1] to give an organoborane [3] .
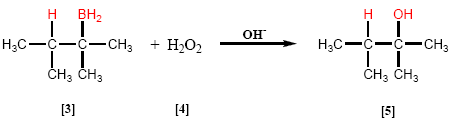
In the second stage, the organoborane [3] is oxidized by treatment with hydrogen peroxide [4] in a basic medium to form the alcohol [5].
The most widely used hydroborating agent is the borane-tetrahydrofuran complex. 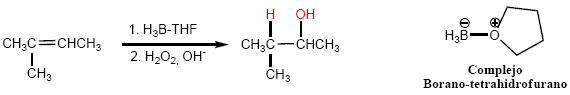
Hydroboration is a reaction with regioselectivity opposite to Markovnikov's rule ( antiMarkovnikov ). Hydrogen adds to the carbon with the fewest hydrogens, and the hydroxyl group goes to the carbon with the most hydrogens. 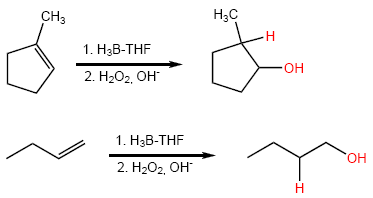
![]() Hydroboration is a SIN stereospecific and regioselective anti-Markovnikov reaction.
Hydroboration is a SIN stereospecific and regioselective anti-Markovnikov reaction.