Hydrogen halides add to alkenes, forming haloalkanes. The proton acts as an electrophile, being attacked by the alkene in the first stage. HF, HCl, HBr, HI can be used as reagents in this reaction.
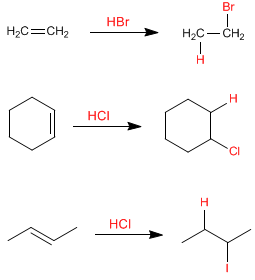
In these examples the alkene is symmetric and it does not matter which carbon of the alkene the hydrogen bonds to. In asymmetric alkenes two types of products can be given depending on which sp 2 carbon the hydrogen is added to. Let's see an example:
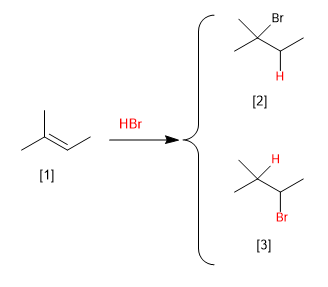
[1] 2-Methyl-2-butene
[2] 2-Bromo-2-methylbutane
[3] 2-Bromo-3-methylbutane (Does not form)
The addition of HBr to 2-methyl-2-butene can generate two products, depending on whether the hydrogen is added to the methyl carbon or to the neighboring one. Experimentally it is observed that 2-bromo-2-methylbutane is obtained and 2-bromo-3-methylbutane does not appear as a product of the reaction.
How can this experimental fact be explained? The answer lies in the reaction mechanism, which we will specify below.
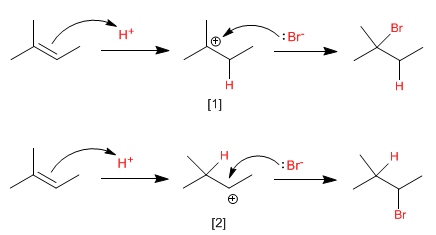
The limiting stage of this mechanism is the electrophilic attack on the proton (first step), in this stage a very unstable reaction intermediate is formed, called carbocation.
The greater stability of the (tertiary) carbocation, compared to that of the (secondary) carbocation, makes the first mechanism more favorable than the second.