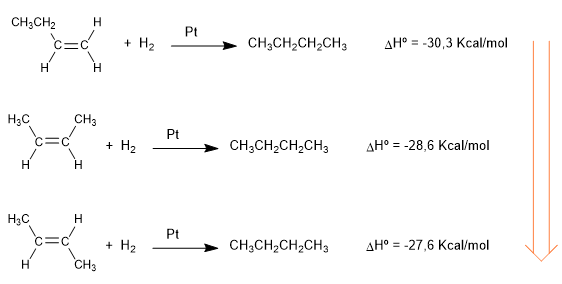
The heats released in the following hydrogenation reactions give us an idea about the different stability of alkenes.
Increased stability
All three alkenes hydrogenate to give the same alkane (butane). 1-butene is the alkene that releases the most energy in hydrogenation, therefore, it is the most unstable (it has more energy). The cis and trans -2-butene have greater stability because they are more substituted alkenes. The interaction between the chains that surround the alkene and the double bond (hyperconjugation) stabilizes it, lowering its energy.
As can be seen, cis -2-butene is more unstable than trans , due to steric repulsions between methyls.
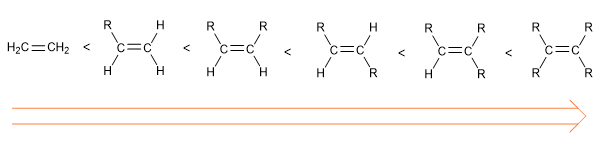
The order of stability of alkenes is as follows:
Increased stability