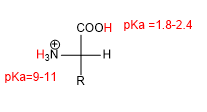
 The presence of acid (-COOH) and basic (-NH 2 ) groups gives amino acids characteristic acid-base properties.
The presence of acid (-COOH) and basic (-NH 2 ) groups gives amino acids characteristic acid-base properties.
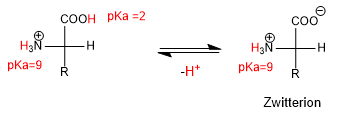
In strong acid media, both the amino group and the acid group are protonated and the amino acid has the following form:
As the pH rises, the more acidic group, H with a lower pKa, is deprotonated, forming a neutral species called Zwitterion.
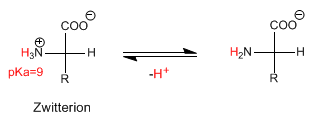
When the amino acid is in basic media, it loses the proton of the amino group, giving rise to the deprotonated species.
The pH at which the Zwitterion concentration is maximum (the amino acid has no net charge) is called isoelectric pH or isoelectric point.
Other definition of isoelectric point is: pH at which the concentration of protonated and deprotonated species equalize.
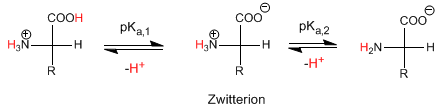
The isoelectric pH is calculated as the average of pK a,1 and pK a,2 , that is, the average of the pKas of the stages that form and decompose the Zwitterion.