Solvents can be classified according to the following scheme. 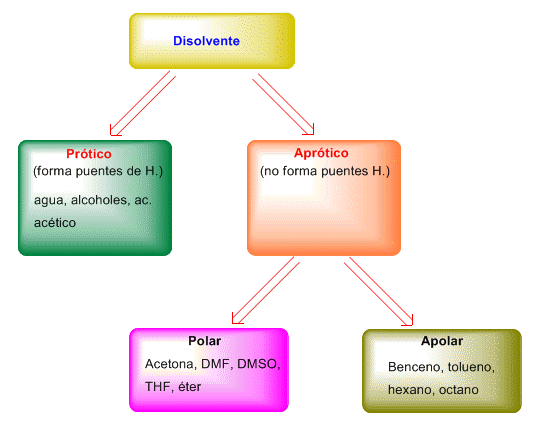
The most suitable solvents for SN2 are the polar aprotic ones. These solvents manage to dissolve the reagents, which are generally very polar, but they do not surround the nucleophile much, leaving it free to attack the substrate. An SN2 in a protic solvent, such as water, alcohols, is thousands of times slower than in an aprotic solvent.
Apolar aprotic solvents are not used due to the poor solubility of both substrate and nucleophile in this type of solvent. 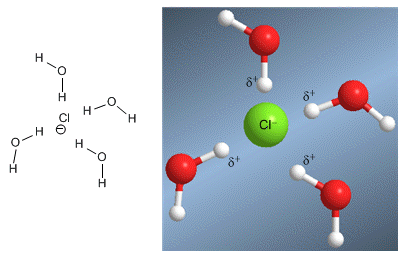
Hydrogen bonds surround chloride ions, decreasing their nucleophilicity.