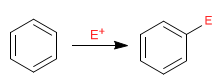
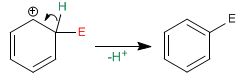Benzene acts as a nucleophile, attacking a large and varied number of electrophiles.
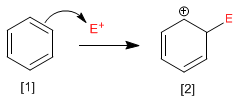
Stage 1. In the first stage of the reaction, the electrophile accepts a pair of electrons from the p cloud of benzene, forming a resonance-stabilized carbocation.
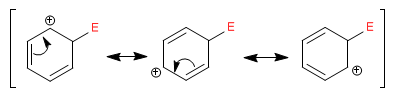
The cyclohexadienyl cation delocalizes the positive charge according to the following structures:
Stage 2. In the second stage, benzene recovers its aromaticity due to the loss of a proton. It is a rapid stage known as rearomatization of the ring.