For the SN1 mechanism to take place, the formation of a stable carbocation is necessary, to allow the dissociation of the substrate.
The stability of a carbocation depends on the number of alkyl groups attached to the carbon that bears the positive charge. Thus, the primary carbocations are less stable than the secondary ones and these in turn less stable than the tertiary ones.
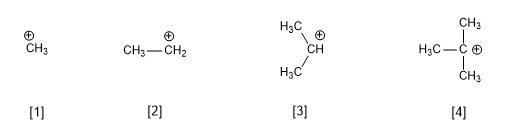
[1] Methyl cation
[2] Ethyl cation (primary)
[3] Isopropyl cation (secondary)
[3] tert-Butyl cation (tertiary)
The instability of the methyl cation and primary carbocations means that the SN1 mechanism does not occur with methyl halides or with primary substrates.
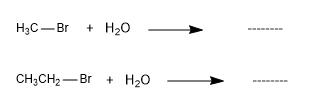
These reactions do not take place, since they form unstable cations (methyl and ethyl)
The phenomenon whereby alkyl groups stabilize carbocations is called hyperconjugation. Hyperconjugation can be defined as the interaction between the carbon-hydrogen bond of the chain and the empty orbital of the positive carbon. This interaction involves a transfer of charge to the carbocation that stabilizes it.
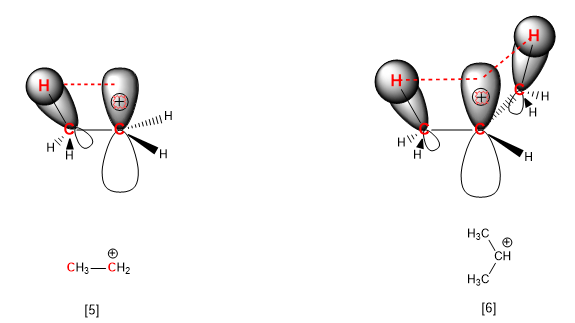
[5]Ethyl cation, with a single interaction by hyperconjugation ( --------- ).
[6] Isopropyl cation with two interactions by hyperconjugation ( --------- ).