The SN1 has a staged mechanism. In the first step, the substrate is ionized by loss of the leaving group, without the nucleophile acting, forming a carbocation. In the second step, the nucleophile attacks the carbocation formed, obtaining the final product. ![]()
Stage 1. Dissociation of the substrate, to form the carbocation. It is the slow pace of the reaction. ![]()
Stage 2. Attack of the nucleophile to the formed carbocation. 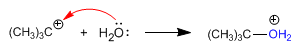
Stage 3. Deprotonation of water to form alcohol 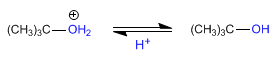
The slow stage of the mechanism is the formation of the carbocation, the speed depending exclusively on the substrate.
v = k[(CH3)3CBr]