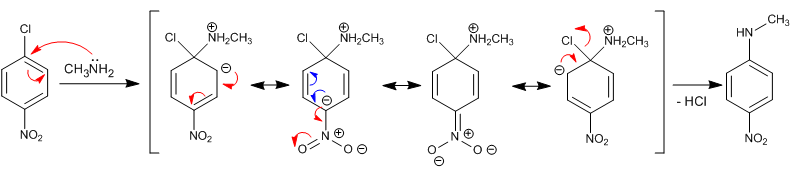
Explain the mechanism of the following reaction:
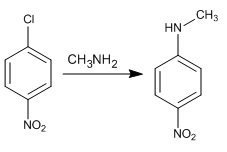
Solution:
When benzene possesses strong deactivating groups in ortho/para with respect to a leaving group, aromatic nucleophilic substitution occurs by addition elimination. This reaction replaces the halogen with the nucleophile. In the first step, the nucleophile attacks the chlorine carbon, delocalizing the charge along the aromatic ring to end up removing the halogen. The reaction places the nucleophile (amine) in the same position as the halogen.