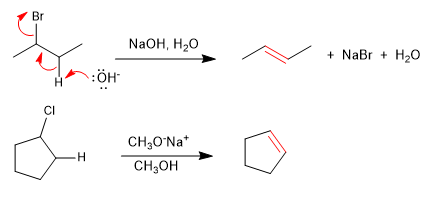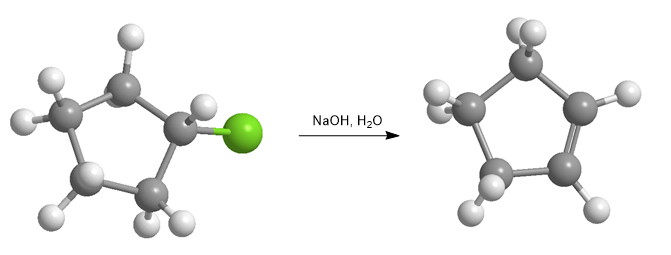
Haloalkanes react with strong bases to form alkenes.
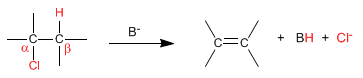
In bimolecular elimination (E2) the base removes hydrogens from the beta carbon and at the same time the leaving group is lost. It is therefore an elementary reaction, whose kinetics is second order.
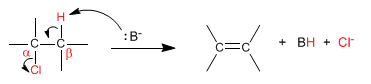
The speed of the reaction depends on the concentration of the base and the substrate.
v = k [substrate][Base]
The energy diagram has the following form:
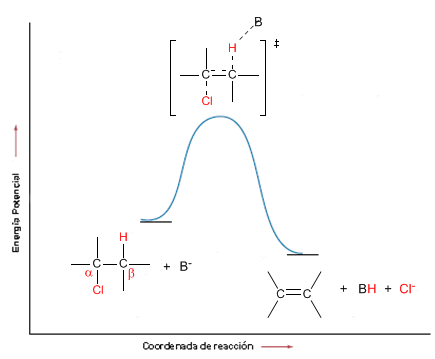
Some examples of bimolecular elimination reactions are: