Nucleophiles are Lewis bases that attack a carbon, displacing the leaving group. Ionic nucleophiles are common, but there are also numerous examples of neutral nucleophiles. The general characteristic of all nucleophiles is the presence of lone pairs on the attacking atom.
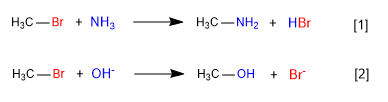
[1] Slow
[2] Fast
In the first reaction, ammonia acts as a nucleophile -neutral species, but with lone pairs on nitrogen- displacing bromine from carbon and forming an amine.
In the second reaction, the hydroxide ion acts as a nucleophile, displacing bromine and forming an alcohol. It is observed that the first reaction is slower than the second, in conclusion ammonia is a worse nucleophile than the hydroxide ion.
The ability of a nucleophile to attack a substrate is known as nucleophilicity. Nucleophilicity depends on several factors: position of the attacking atom in the periodic table, charge, and resonance.
Charge - Charged species are better nucleophiles than neutral ones
OH- >> H2O Hydroxide ion (charged species) better nucleophile than water (neutral species)
NH2- >> NH3 Amide ion (charged species) better nucleophile than ammonia (neutral species)
PH 2- >> PH3 Phosphide ion (charged species) better nucleophile than phosphine (neutral species)
Position on the periodic table - Nucleophilicity increases going down the periodic table and moving to the left.
NH3 > H2O Ammonia is a better nucleophile than water because nitrogen is more to the left than oxygen
I- > Cl- Iodide is a better nucleophile than chloride because it is lower down.
PH3 > NH3 Phosphine is a better nucleophile than ammonia because phosphorus is lower than nitrogen.
Resonance - Resonance decreases nucleophilicity. Delocalization of lone pairs decreases the ability of the nucleophile to attack.
OH- > CH3COO- The acetate ion is worse nucleophilic than the hydroxide ion due to charge delocalization on both oxygens.