The SN2 (bimolecular nucleophilic substitution) is a concerted reaction, that is, it occurs in a single step.
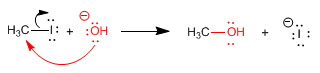
The mechanism consists of the nucleophile attacking the carbon containing the leaving group. This carbon presents an important positive polarity, due to the electronegativity of the halogen. At the same time that the nucleophile attacks, the carbon-halogen bond is broken, obtaining the final product.
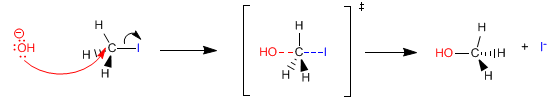
The transition state of SN2 has the following form:
The rate of an elementary reaction is proportional to the product of the concentrations of the reactants raised to their respective stoichiometric coefficients. The constant of proportionality is called the kinetic constant.
Thus, the speed of SN2 depends on the concentration of the substrate (CH3I) and the nucleophile (OH- ), and is therefore a bimolecular reaction.
v=k[CH 3I][OH-]