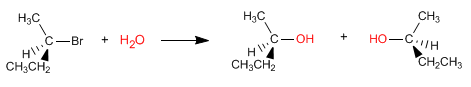
The SN1 reaction proceeds through a planar carbocation, which is attacked by the nucleophile on both sides, giving rise to a mixture of stereoisomers.
Stage 1. Dissociation of the substrate, forming the planar carbocation
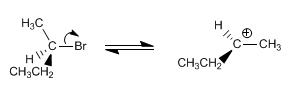
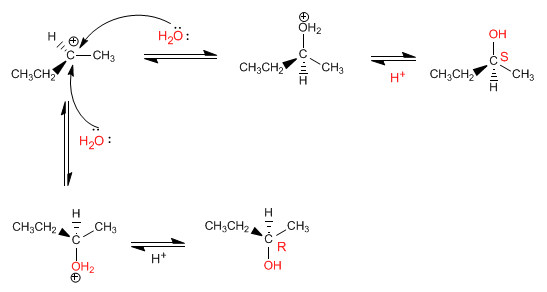
Stage 2. Attack of the nucleophile on both sides giving rise to the formation of two enantiomers in equal proportion (racemic mixture).
The products formed are enantiomers and are obtained in equal amounts, since the two faces of the carbocation are indistinguishable for water.